A cyanostar (pentacyanopentabenzo[25]annulene) is a shape-persistent macrocycle that binds anions. [1] [2]
Contents

A cyanostar (pentacyanopentabenzo[25]annulene) is a shape-persistent macrocycle that binds anions. [1] [2]

The cyanostar structure is synthesized in a one-pot process among five equivalents of a benzaldehyde bearing a meta -cyanomethyl substituent. A series of Knoevenagel condensation reactions catalyzed by various bases stitches them together to make the C5-symmetric structure. [3]
Cyanostar binds anions through hydrogen bonding from the C–H hydrogen bonds, as the hydrogen has a slight positive charge. [3] It is the first binder to make use of cyanostilbene's electropositive CH groups. The hydrogen bonds create an electropositive region in the center of the macrocycle, creating a binding pocket. Cyanostar strongly binds anions that usually can only be bound weakly. The increased binding arises from the formation of a 2:1 complex, with two cyanostars sandwiching the anion on each side. [3] An extended version of this structural pattern is a 4:3 alternating stack of cyanostar molecules complexing a hydrogen-bonded chain of dihydrogen phosphate units. [4]
Two cyanostars can be threaded onto a phosphate diester structure, forming a rotaxane. Because they have a high affinity for the central phosphate group only when it is in its anionic form, there is a substantial and reversible structural change in response to acid–base changes in solution. [5]

A hydrogen bond is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative atom or group, particularly the second-row elements nitrogen (N), oxygen (O), or fluorine (F)—the hydrogen bond donor (Dn)—and another electronegative atom bearing a lone pair of electrons—the hydrogen bond acceptor (Ac). Such an interacting system is generally denoted Dn–H···Ac, where the solid line denotes a fully covalent bond, and the dotted or dashed line indicates the hydrogen bond. The use of three centered dots for the hydrogen bond is specifically recommended by the IUPAC. There is general agreement that there is actually a minor covalent component to hydrogen bonding, especially for moderate to strong hydrogen bonds, although the importance of covalency in hydrogen bonding is debated. At the opposite end of the scale, there is no clear boundary between a weak hydrogen bond and a van der Waals interaction.

A rotaxane is a mechanically interlocked molecular architecture consisting of a "dumbbell shaped molecule" which is threaded through a "macrocycle". The name is derived from the Latin for wheel (rota) and axle (axis). The two components of a rotaxane are kinetically trapped since the ends of the dumbbell are larger than the internal diameter of the ring and prevent dissociation (unthreading) of the components since this would require significant distortion of the covalent bonds.
A carbanion is an anion in which carbon is trivalent (forms three bonds) and bears a formal negative charge (in at least one significant resonance form). Absent π delocalization, carbanions assume a trigonal pyramidal, bent, or linear geometry when the carbanionic carbon is bound to three (e.g., methyl anion), two (e.g., phenyl anion), or one (e.g., acetylide anion) substituents, respectively. Formally, a carbanion is the conjugate base of a carbon acid:

Crown ethers are cyclic chemical compounds that consist of a ring containing several ether groups. The most common crown ethers are cyclic oligomers of ethylene oxide, the repeating unit being ethyleneoxy, i.e., –CH2CH2O–. Important members of this series are the tetramer (n = 4), the pentamer (n = 5), and the hexamer (n = 6). The term "crown" refers to the resemblance between the structure of a crown ether bound to a cation, and a crown sitting on a person's head. The first number in a crown ether's name refers to the number of atoms in the cycle, and the second number refers to the number of those atoms that are oxygen. Crown ethers are much broader than the oligomers of ethylene oxide; an important group are derived from catechol.
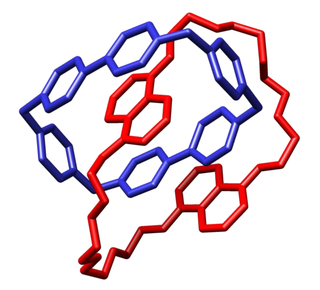
A catenane is a mechanically-interlocked molecular architecture consisting of two or more interlocked macrocycles, i.e. a molecule containing two or more intertwined rings. The interlocked rings cannot be separated without breaking the covalent bonds of the macrocycles. Catenane is derived from the Latin catena meaning "chain". They are conceptually related to other mechanically interlocked molecular architectures, such as rotaxanes, molecular knots or molecular Borromean rings. Recently the terminology "mechanical bond" has been coined that describes the connection between the macrocycles of a catenane. Catenanes have been synthesised in two different ways: statistical synthesis and template-directed synthesis.
Anions that interact weakly with cations are termed non-coordinating anions, although a more accurate term is weakly coordinating anion. Non-coordinating anions are useful in studying the reactivity of electrophilic cations. They are commonly found as counterions for cationic metal complexes with an unsaturated coordination sphere. These special anions are essential components of homogeneous olefin polymerisation catalysts, where the active catalyst is a coordinatively unsaturated, cationic transition metal complex. For example, they are employed as counterions for the 14 valence electron cations [(C5H5)2ZrR]+ (R = methyl or a growing polyethylene chain). Complexes derived from non-coordinating anions have been used to catalyze hydrogenation, hydrosilylation, oligomerization, and the living polymerization of olefins. The popularization of non-coordinating anions has contributed to increased understanding of agostic complexes wherein hydrocarbons and hydrogen serve as ligands. Non-coordinating anions are important components of many superacids, which result from the combination of Brønsted acids and Lewis acids.

Cucurbiturils are macrocyclic molecules made of glycoluril (=C4H2N4O2=) monomers linked by methylene bridges (-CH2-). The oxygen atoms are located along the edges of the band and are tilted inwards, forming a partly enclosed cavity. The name is derived from the resemblance of this molecule with a pumpkin of the family of Cucurbitaceae.
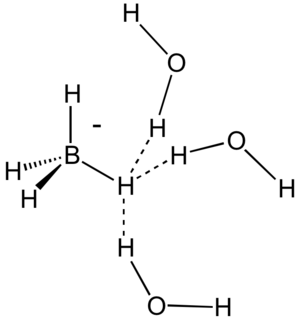
In chemistry, a dihydrogen bond is a kind of hydrogen bond, an interaction between a metal hydride bond and an OH or NH group or other proton donor. With a van der Waals radius of 1.2 Å, hydrogen atoms do not usually approach other hydrogen atoms closer than 2.4 Å. Close approaches near 1.8 Å, are, however, characteristic of dihydrogen bonding.

Macrocycles are often described as molecules and ions containing twelve or more membered ring. Classical examples include the crown ethers, calixarenes, porphyrins, and cyclodextrins. Macrocycles describe a large, mature area of chemistry.
A non-covalent interaction differs from a covalent bond in that it does not involve the sharing of electrons, but rather involves more dispersed variations of electromagnetic interactions between molecules or within a molecule. The chemical energy released in the formation of non-covalent interactions is typically on the order of 1-5 kcal/mol. Non-covalent interactions can be classified into different categories, such as electrostatic, π-effects, van der Waals forces, and hydrophobic effects.

In chemistry, a foldamer is a discrete chain molecule or oligomer that folds into a conformationally ordered state in solution. They are artificial molecules that mimic the ability of proteins, nucleic acids, and polysaccharides to fold into well-defined conformations, such as helices and β-sheets. The structure of a foldamer is stabilized by noncovalent interactions between nonadjacent monomers. Foldamers are studied with the main goal of designing large molecules with predictable structures. The study of foldamers is related to the themes of molecular self-assembly, molecular recognition, and host–guest chemistry.
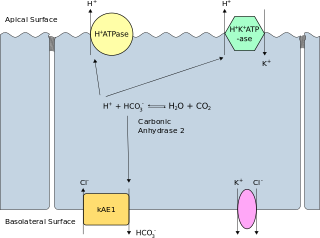
Band 3 anion transport protein, also known as anion exchanger 1 (AE1) or band 3 or solute carrier family 4 member 1 (SLC4A1), is a protein that is encoded by the SLC4A1 gene in humans.
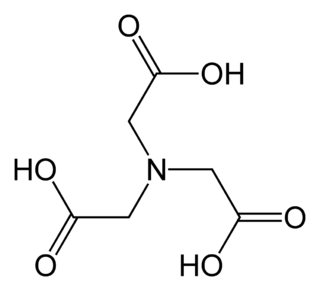
Nitrilotriacetic acid (NTA) is the aminopolycarboxylic acid with the formula N(CH2CO2H)3. It is a colourless solid that is used as a chelating agent, which forms coordination compounds with metal ions (chelates) such as Ca2+, Co2+, Cu2+, and Fe3+.
Carbon–hydrogen bond functionalization is a type of reaction in which a carbon–hydrogen bond is cleaved and replaced with a carbon–X bond. The term usually implies that a transition metal is involved in the C-H cleavage process. Reactions classified by the term typically involve the hydrocarbon first to react with a metal catalyst to create an organometallic complex in which the hydrocarbon is coordinated to the inner-sphere of a metal, either via an intermediate "alkane or arene complex" or as a transition state leading to a "M−C" intermediate. The intermediate of this first step can then undergo subsequent reactions to produce the functionalized product. Important to this definition is the requirement that during the C–H cleavage event, the hydrocarbyl species remains associated in the inner-sphere and under the influence of "M".
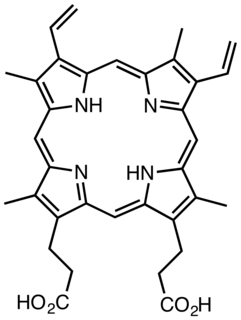
Protoporphyrin IX is an organic compound, specifically a porphyrin, that plays an important role in living organisms as a precursor other critical compounds like hemoglobin and chlorophyll. It is a deeply colored solid that is not soluble in basic water. The name is often abbreviated as PPIX.
In chemistry, π-effects or π-interactions are a type of non-covalent interaction that involves π systems. Just like in an electrostatic interaction where a region of negative charge interacts with a positive charge, the electron-rich π system can interact with a metal, an anion, another molecule and even another π system. Non-covalent interactions involving π systems are pivotal to biological events such as protein-ligand recognition.

The Birch reduction is an organic reaction that is used to convert arenes to cyclohexadienes. The reaction is named after the Australian chemist Arthur Birch. In this organic reduction of aromatic rings in liquid ammonia with sodium, lithium, or potassium and an alcohol, such as ethanol and tert-butanol. This reaction is unlike catalytic hydrogenation, which usually reduces the aromatic ring all the way to a cyclohexane.
Indium trihydride is an inorganic compound with the chemical formula n. It is a covalent network solid, and as such, it is insoluble in all solvents. Moreover, it is unstable at standard temperature and pressure. It is a group 13 hydride.
Asymmetric ion-pairing catalysis in chemistry is a type of asymmetric catalysis taking place specifically with charged intermediates or charged reagents. In one type of catalysis ion-pairing exists with a charged and chiral catalyst. The charged catalyst can be cationic or anionic. Catalysis by anionic catalysts is also called asymmetric counteranion-directed catalysis. In the other variation of asymmetric ion-pairing catalysis called anion or cation binding, the chiral catalyst is neutral but binds in a noncovalent way to an intermediate ion pair. Asymmetric ion-pairing catalysis is distinct from other covalent types of catalys such as Lewis acid catalysis and Bronsted acid catalysis. It is one of several strategies in enantioselective synthesis and of some relevance to academic research.
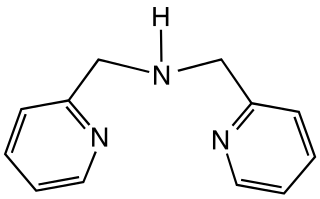
Dipicolylamine is an organic compound with the formula HN(CH2C5H4N)2. It is a yellow liquid that is soluble in polar organic solvents. The molecule is a secondary amine with two picolyl substituents. The compound is a common tridentate ligand in coordination chemistry.