A cycloalkene or cycloolefin is a type of alkene hydrocarbon which contains a closed ring of carbon atoms, but has no aromatic character. Some cycloalkenes, such as cyclobutene and cyclopentene, can be used as monomers to produce polymer chains. Due to geometrical considerations, smaller cycloalkenes are almost always the cis isomers, and the term cis tends to be omitted from the names. In larger rings, cis–trans isomerism of the double bond may occur.
1,3-Cyclohexadiene is an organic compound with the formula (CH2)2(CH)4. It is a colorless, flammable liquid. Its refractive index is 1.475 (20 °C, D). A naturally occurring derivative of 1,3-cyclohexadiene is terpinene, a component of pine oil.
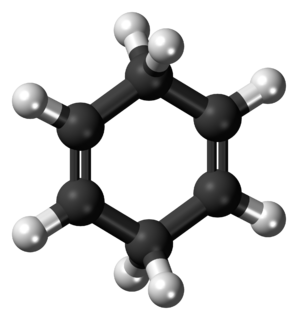
1,4-Cyclohexadiene is an organic compound with the formula C6H8. It is a colourless, flammable liquid that is of academic interest as a prototype of a large class of related compounds called terpenoids, an examples being γ-terpinene. An isomer exists of this compound, 1,3-cyclohexadiene.
Sodium amalgam, commonly denoted Na(Hg), is an alloy of mercury and sodium. The term amalgam is used for alloys, intermetallic compounds, and solutions involving mercury as a major component. Sodium amalgam is often used in reactions as a strong reducing agent with better handling properties compared to solid sodium. They are less dangerously reactive toward water and in fact are often used as an aqueous suspension.

Prephenic acid, commonly also known by its anionic form prephenate, is an intermediate in the biosynthesis of the aromatic amino acids phenylalanine and tyrosine, as well as of a large number of secondary metabolites of the shikimate pathway.

Isoindene is a flammable polycyclic hydrocarbon with chemical formula C9H8. It is composed of a cyclohexadiene ring fused with a cyclopentadiene ring.

Ruthenium(III) chloride is the chemical compound with the formula RuCl3. "Ruthenium(III) chloride" more commonly refers to the hydrate RuCl3·xH2O. Both the anhydrous and hydrated species are dark brown or black solids. The hydrate, with a varying proportion of water of crystallization, often approximating to a trihydrate, is a commonly used starting material in ruthenium chemistry. The compound is also widely regarded as a prime candidate to realize Kitaev quantum spin liquid state with Majorana Fermion excitations.
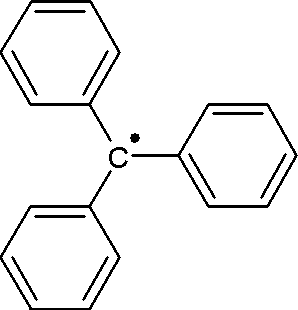
The triphenylmethyl radical is a persistent radical and the first radical ever described in organic chemistry. It can be prepared by homolysis of triphenylmethyl chloride 1 by a metal like silver or zinc in benzene or diethyl ether. The radical 2 forms a chemical equilibrium with the quinoid type dimer 3 (3-triphenylmethyl-6-diphenylmethylidene-1,4-cyclohexadiene). In benzene the concentration of the radical is 2%.
The Cadiot–Chodkiewicz coupling in organic chemistry is a coupling reaction between a terminal alkyne and a haloalkyne catalyzed by a copper(I) salt such as copper(I) bromide and an amine base. The reaction product is a 1,3-diyne or di-alkyne.

The isotoluenes in organic chemistry are the non-aromatic toluene isomers with an exocyclic double bond. They are of some academic interest in relation to aromaticity and isomerisation mechanisms.

Tetralin (1,2,3,4-tetrahydronaphthalene) is a hydrocarbon having the chemical formula C10H12. This molecule is similar to the naphthalene chemical structure except that one ring is saturated.
The molecular formula C6H8 may refer to:
In enzymology, a 1,6-dihydroxycyclohexa-2,4-diene-1-carboxylate dehydrogenase (EC 1.3.1.25) is an enzyme that catalyzes the chemical reaction
In enzymology, a (3S,4R)-3,4-dihydroxycyclohexa-1,5-diene-1,4-dicarboxylate dehydrogenase (EC 1.3.1.53) is an enzyme that catalyzes the chemical reaction
Organoiron chemistry is the chemistry of iron compounds containing a carbon-to-iron chemical bond. Organoiron compounds are relevant in organic synthesis as reagents such as iron pentacarbonyl, diiron nonacarbonyl and disodium tetracarbonylferrate. Iron adopts oxidation states from Fe(−II) through to Fe(VII). Although iron is generally less active in many catalytic applications, it is less expensive and "greener" than other metals. Organoiron compounds feature a wide range of ligands that support the Fe-C bond; as with other organometals, these supporting ligands prominently include phosphines, carbon monoxide, and cyclopentadienyl, but hard ligands such as amines are employed as well.

Chloranil is a quinone with the molecular formula C6Cl4O2. Also known as tetrachloro-1,4-benzoquinone, it is a yellow solid. Like the parent benzoquinone, chloranil is a planar molecule that functions as a mild oxidant.

The Birch reduction is an organic reaction which is particularly useful in synthetic organic chemistry. The reaction was reported in 1944 by the Australian chemist Arthur Birch (1915–1995) working in the Dyson Perrins Laboratory at the University of Oxford, building on earlier work by Wooster and Godfrey published in 1937. It converts aromatic compounds having a benzenoid ring into a product, 1,4-cyclohexadienes, in which two hydrogen atoms have been attached on opposite ends of the molecule. It is the organic reduction of aromatic rings in liquid ammonia with sodium, lithium or potassium and an alcohol, such as ethanol and tert-butanol. This reaction is quite unlike catalytic hydrogenation, which usually reduces the aromatic ring all the way to a cyclohexane.
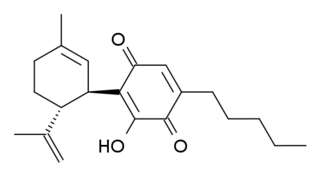
HU-331 is a quinone anticarcinogenic drug synthesized from cannabidiol, a cannabinoid in the Cannabis sativa plant. It showed a great efficacy against oncogenic human cells. HU-331 does not cause arrest in cell cycle, cell apoptosis or caspase activation. HU-331 inhibits DNA topoisomerase II even at nanomolar concentrations, but has shown a negligible effect on the action of DNA topoisomerase I. The cannabinoid quinone HU-331 is a very specific inhibitor of topoisomerase II, compared with most known anticancer quinones. One of the main objectives of these studies is the development of a new quinone derived compound that produces anti-neoplastic activity while maintaining low toxicity at therapeutic doses.
Ganugapati Sree Rama Subba Rao is an Indian natural product chemist and a former chair of the department of sciences at the Indian Institute of Science (IISc). He is known for his researches on dihydroaromatics obtained through Birch reduction of aromatic compounds and is an elected fellow of the Indian National Science Academy, and the Indian Academy of Sciences. The Council of Scientific and Industrial Research, the apex agency of the Government of India for scientific research, awarded him the Shanti Swarup Bhatnagar Prize for Science and Technology, one of the highest Indian science awards, in 1982, for his contributions to chemical sciences.










