
Rust is an iron oxide, a usually reddish brown oxide formed by the reaction of iron and oxygen in the presence of water or air moisture. Several forms of rust are distinguishable both visually and by spectroscopy, and form under different circumstances. Rust consists of hydrated iron(III) oxides Fe2O3·nH2O and iron(III) oxide-hydroxide (FeO(OH), Fe(OH)3).

Corrosion is a natural process that converts a refined metal into a more chemically-stable form such as oxide, hydroxide, or sulfide. It is the gradual destruction of materials by chemical and/or electrochemical reaction with their environment. Corrosion engineering is the field dedicated to controlling and preventing corrosion.
Chelation is a type of bonding of ions and molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate ligand and a single central atom. These ligands are called chelants, chelators, chelating agents, or sequestering agents. They are usually organic compounds, but this is not a necessity, as in the case of zinc and its use as a maintenance therapy to prevent the absorption of copper in people with Wilson's disease.

Ethylenediaminetetraacetic acid (EDTA), also known by several other names, is a chemical used for both industrial and medical purposes. It was synthesized for the first time in 1935 by Ferdinand Münz.

An antifreeze is an additive which lowers the freezing point of a water-based liquid. An antifreeze mixture is used to achieve freezing-point depression for cold environments. Common antifreezes increase the boiling point of the liquid, allowing higher coolant temperature.
Phosphorous acid, is the compound described by the formula H3PO3. This acid is diprotic (readily ionizes two protons), not triprotic as might be suggested by this formula. Phosphorous acid is an intermediate in the preparation of other phosphorus compounds. Organic derivatives of phosphorous acid, compounds with the formula RPO3H2, are called phosphonic acids.

A phosphoric acid, in the general sense, is a phosphorus oxoacid in which each phosphorus atom is in the oxidation state +5, and is bonded to four oxygen atoms, one of them through a double bond, arranged as the corners of a tetrahedron. Two or more of these PO
4 tetrahedra may be connected by shared single-bonded oxygens, forming linear or branched chains, cycles, or more complex structures. The single-bonded oxygen atoms that are not shared are completed with acidic hydrogen atoms. The general formula of a phosphoric acid is Hn+2−2xPnO3n+1−x, where n is the number of phosphorus atoms and x is the number of fundamental cycles in the molecule's structure, between 0 and (n+2)/2.
A corrosion inhibitor is a chemical compound that, when added to a liquid or gas, decreases the corrosion rate of a material, typically a metal or an alloy, that comes into contact with the fluid. The effectiveness of a corrosion inhibitor depends on fluid composition, quantity of water, and flow regime. Corrosion inhibitors are common in industry, and also found in over-the-counter products, typically in spray form in combination with a lubricant and sometimes a penetrating oil. They may be added to water to prevent leaching of lead or copper from pipes.
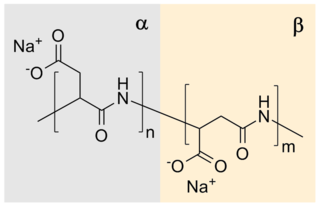
Sodium polyaspartate is a sodium salt of polyaspartic acid. It is biodegradable condensation polymer based on the amino acid aspartic acid.

Fouling is the accumulation of unwanted material on solid surfaces to the detriment of function. The fouling materials can consist of either living organisms (biofouling) or a non-living substance. Fouling is usually distinguished from other surface-growth phenomena in that it occurs on a surface of a component, system, or plant performing a defined and useful function and that the fouling process impedes or interferes with this function.

Phosphonates and phosphonic acids are organophosphorus compounds containing C−PO(OH)2 or C−PO(OR)2 groups (where R = alkyl, aryl). Phosphonic acids, typically handled as salts, are generally nonvolatile solids that are poorly soluble in organic solvents, but soluble in water and common alcohols. Many commercially important compounds are phosphonates, including glyphosate (the active molecule of the herbicide "Roundup"), and ethephon, a widely used plant growth regulator. Bisphosphonates are popular drugs for treatment of osteoporosis.
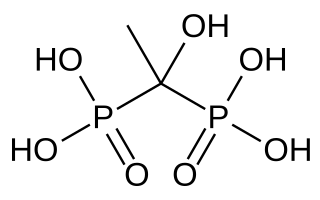
Etidronic acid, also known as etidronate, is a bisphosphonate used as a medication, detergent, water treatment, and cosmetic.

Pentetic acid or diethylenetriaminepentaacetic acid (DTPA) is an aminopolycarboxylic acid consisting of a diethylenetriamine backbone with five carboxymethyl groups. The molecule can be viewed as an expanded version of EDTA and is used similarly. It is a white solid with limited solubility in water.

Boiler feedwater is an essential part of boiler operations. The feed water is put into the steam drum from a feed pump. In the steam drum the feed water is then turned into steam from the heat. After the steam is used it is then dumped to the main condenser. From the condenser it is then pumped to the deaerated feed tank. From this tank it then goes back to the steam drum to complete its cycle. The feed water is never open to the atmosphere. This cycle is known as a closed system or Rankine cycle.
Cleaning agents or hard-surface cleaners are substances used to remove dirt, including dust, stains, bad smells, and clutter on surfaces. Purposes of cleaning agents include health, beauty, removing offensive odor, and avoiding the spread of dirt and contaminants to oneself and others. Some cleaning agents can kill bacteria and clean at the same time. Others, called degreasers, contain organic solvents to help dissolve oils and fats.
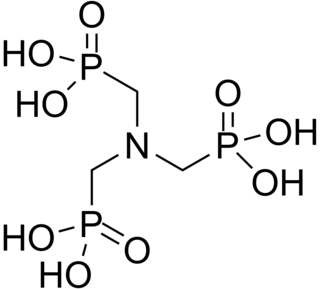
ATMP or aminotris(methylenephosphonic acid) is a phosphonic acid with chemical formula N(CH2PO3H2)3. It has chelating properties. It can be synthesized from the Mannich-type reaction of ammonia, formaldehyde, and phosphorous acid, in a manner similar to the Kabachnik–Fields reaction.

EDTMP or ethylenediamine tetra(methylene phosphonic acid) is a phosphonic acid. It has chelating and anti corrosion properties. EDTMP is the phosphonate analog of EDTA. It is classified as a nitrogenous organic polyphosphonic acid.
Calcium nitrite is an inorganic compound with chemical formula Ca(NO2)2. In this compound, as in all nitrites, nitrogen is in a +3 oxidation state. It has many applications such as antifreeze, rust inhibitor of steel and wash heavy oil.
Oilfield scale inhibition is the process of preventing the formation of scale from blocking or hindering fluid flow through pipelines, valves, and pumps used in oil production and processing. Scale inhibitors (SIs) are a class of specialty chemicals that are used to slow or prevent scaling in water systems. Oilfield scaling is the precipitation and accumulation of insoluble crystals (salts) from a mixture of incompatible aqueous phases in oil processing systems. Scale is a common term in the oil industry used to describe solid deposits that grow over time, blocking and hindering fluid flow through pipelines, valves, pumps etc. with significant reduction in production rates and equipment damages. Scaling represents a major challenge for flow assurance in the oil and gas industry. Examples of oilfield scales are calcium carbonate (limescale), iron sulfides, barium sulfate and strontium sulfate. Scale inhibition encompasses the processes or techniques employed to treat scaling problems.
N-Oleoylsarcosine is an amphiphilic oleic acid derivative having a sarcosine head group (N-methylglycine) which is used as a water-in-oil emulsifier and corrosion inhibitor.













