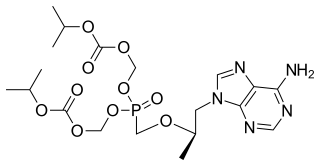
Tenofovir disoproxil, sold under the trade name Viread among others, is a medication used to treat chronic hepatitis B and to prevent and treat HIV/AIDS. It is generally recommended for use with other antiretrovirals. It may be used for prevention of HIV/AIDS among those at high risk before exposure, and after a needlestick injury or other potential exposure. It is sold both by itself and together in combinations such as emtricitabine/tenofovir, efavirenz/emtricitabine/tenofovir, and elvitegravir/cobicistat/emtricitabine/tenofovir. It does not cure HIV/AIDS or hepatitis B. It is available by mouth as a tablet or powder.
Efavirenz (EFV), sold under the brand names Sustiva among others, is an antiretroviral medication used to treat and prevent HIV/AIDS. It is generally recommended for use with other antiretrovirals. It may be used for prevention after a needlestick injury or other potential exposure. It is sold both by itself and in combination as efavirenz/emtricitabine/tenofovir. It is taken by mouth.

Darunavir (DRV), sold under the brand name Prezista among others, is an antiretroviral medication used to treat and prevent HIV/AIDS. It is generally recommended for use with other antiretrovirals. It is often used with low doses of ritonavir or cobicistat to increase darunavir levels. It may be used for prevention after a needlestick injury or other potential exposure. It is taken by mouth once to twice a day.

Efavirenz/emtricitabine/tenofovir, sold under the brand name Atripla among others, is a fixed-dose combination antiretroviral medication used to treat HIV/AIDS. It contains efavirenz, emtricitabine, and tenofovir disoproxil. It can be used by itself or together with other antiretroviral medications. It is taken by mouth.

Raltegravir, sold under the brand name Isentress, is an antiretroviral medication used, together with other medication, to treat HIV/AIDS. It may also be used, as part of post exposure prophylaxis, to prevent HIV infection following potential exposure. It is taken by mouth.
Integrase inhibitors (INIs) are a class of antiretroviral drug designed to block the action of integrase, a viral enzyme that inserts the viral genome into the DNA of the host cell. Since integration is a vital step in retroviral replication, blocking it can halt further spread of the virus. Integrase inhibitors were initially developed for the treatment of HIV infection, but have been applied to other retroviruses. The class of integrase inhibitors called integrase strand transfer inhibitors (INSTIs) are in established medical use. Other classes, such as allosteric integrase inhibitors (ALLINIs) or integrase binding inhibitors (INBIs), are still experimental.

Rilpivirine, sold under the brand names Edurant and Rekambys, is a medication, developed by Tibotec, used for the treatment of HIV/AIDS. It is a second-generation non-nucleoside reverse transcriptase inhibitor (NNRTI) with higher potency, longer half-life and reduced side-effect profile compared with older NNRTIs such as efavirenz.

Dolutegravir (DTG), sold under the brand name Tivicay, is an antiretroviral medication used, together with other medication, to treat HIV/AIDS. It may also be used, as part of post exposure prophylaxis, to prevent HIV infection following potential exposure. It is taken by mouth.
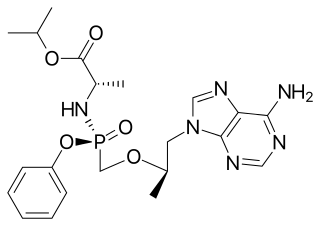
Tenofovir alafenamide, sold under the brand name Vemlidy, is an antiviral medication used against hepatitis B and HIV. It is used for the treatment of chronic hepatitis B virus (HBV) infection in adults with compensated liver disease and is given in combination with other medications for the prevention and treatment of HIV. It is taken by mouth.

Cabotegravir, sold under the brand name Vocabria among others, is a antiretroviral medication used for the treatment of HIV/AIDS. It is available in the form of tablets and as an intramuscular injection, as well as in an injectable combination with rilpivirine under the brand name Cabenuva.

Fostemsavir, sold under the brand name Rukobia, is an antiretroviral medication for adults living with HIV/AIDS who have tried multiple HIV medications and whose HIV infection cannot be successfully treated with other therapies because of resistance, intolerance or safety considerations.

Abacavir/dolutegravir/lamivudine, sold under the brand name Triumeq among others, is a fixed-dose combination antiretroviral medication for the treatment of HIV/AIDS. It is a combination of three medications with different and complementary mechanisms of action: abacavir, dolutegravir and lamivudine.
Efavirenz/lamivudine/tenofovir (EFV/3TC/TDF), sold under the brand name Symfi among others, is a fixed-dose combination antiretroviral medication for the treatment of HIV/AIDS. It combines efavirenz, lamivudine, and tenofovir disoproxil. As of 2019, it is listed by the World Health Organization as an alternative first line option to dolutegravir/lamivudine/tenofovir. It is taken by mouth.
Atazanavir/cobicistat, sold under the brand name Evotaz, is a fixed-dose combination antiretroviral medication used to treat and prevent HIV/AIDS. It contains atazanavir and cobicistat. Atazanavir is an HIV protease inhibitor and cobicistat is an inhibitor of cytochrome P450 (CYP) enzymes of the CYP3A family.
Doravirine/lamivudine/tenofovir, sold under the brand name Delstrigo, is a fixed-dose combination antiretroviral medication for the treatment of HIV/AIDS. It contains doravirine, lamivudine, and tenofovir disoproxil. It is taken by mouth.
Dolutegravir/lamivudine, sold under the brand name Dovato, is a fixed-dose combination antiretroviral medication for the treatment of HIV/AIDS. It contains dolutegravir, as the salt, an integrase strand transfer inhibitor (INSTI), and lamivudine, a nucleoside analogue reverse transcriptase inhibitor (NRTI). It is taken by mouth.
Dapagliflozin/metformin, sold under the brand name Xigduo XR amongst others, is a fixed-dose combination anti-diabetic medication used as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes. It is a combination of dapagliflozin and metformin and is taken by mouth. Dapagliflozin/metformin was approved for use in the European Union in January 2014, in the United States in February 2014, and in Australia in July 2014.
Dolutegravir/rilpivirine, sold under the brand name Juluca, is a fixed-dose combination antiretroviral medication for the treatment of HIV/AIDS. It contains the medicines dolutegravir and rilpivirine. It is taken by mouth.

Cabotegravir/rilpivirine, sold under the brand name Cabenuva, is a co-packaged antiretroviral medication for the treatment of HIV/AIDS. It contains cabotegravir and rilpivirine in a package with two separate injection vials.

Lenacapavir, sold under the brand name Sunlenca, is an antiretroviral medication used to treat HIV/AIDS. It is taken by mouth or by subcutaneous injection.











