
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic organic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of organic heterocycles.

Imidazole (ImH) is an organic compound with the formula C3N2H4. It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. In chemistry, it is an aromatic heterocycle, classified as a diazole, and has non-adjacent nitrogen atoms in meta-substitution.
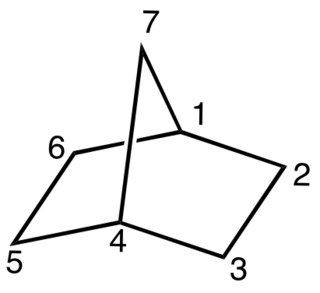
A bicyclic molecule is a molecule that features two joined rings. Bicyclic structures occur widely, for example in many biologically important molecules like α-thujene and camphor. A bicyclic compound can be carbocyclic, or heterocyclic, like DABCO. Moreover, the two rings can both be aliphatic, or can be aromatic, or a combination of aliphatic and aromatic.

Piperazine is an organic compound that consists of a six-membered ring containing two nitrogen atoms at opposite positions in the ring. Piperazine exists as small alkaline deliquescent crystals with a saline taste.

Triazines are a class of nitrogen-containing heterocycles. The parent molecules' molecular formula is C3H3N3. They exist in three isomeric forms, 1,3,5-triazines being common.
In organic chemistry, diazines are a group of organic compounds having the molecular formula C4H4N2. Each contains a benzene ring in which two of the C-H fragments have been replaced by isolobal nitrogen. There are three structural isomers:
A triazole is a heterocyclic compound featuring a five-membered ring of two carbon atoms and three nitrogen atoms with molecular formula C2H3N3. Triazoles exhibit substantial isomerism, depending on the positioning of the nitrogen atoms within the ring.
1,2,3-Triazole is one of a pair of isomeric chemical compounds with molecular formula C2H3N3, called triazoles, which have a five-membered ring of two carbon atoms and three nitrogen atoms. 1,2,3-Triazole is a basic aromatic heterocycle.
Thiazine is an organic compound containing a ring of four carbon, one nitrogen and one sulfur atom. There are three isomers of thiazine, 1,2-thiazine, 1,3-thiazine, and 1,4-thiazine, which differ by the arrangement of the nitrogen and sulfur atoms in the ring.

In organic chemistry, spiro compounds are compounds that have at least two molecular rings with only one common atom. The simplest spiro compounds are bicyclic, or have a bicyclic portion as part of the larger ring system, in either case with the two rings connected through the defining single common atom. The one common atom connecting the participating rings distinguishes spiro compounds from other bicyclics: from isolated ring compounds like biphenyl that have no connecting atoms, from fused ring compounds like decalin having two rings linked by two adjacent atoms, and from bridged ring compounds like norbornane with two rings linked by two non-adjacent atoms.
In organic chemistry, umpolung or polarity inversion is the chemical modification of a functional group with the aim of the reversal of polarity of that group. This modification allows secondary reactions of this functional group that would otherwise not be possible. The concept was introduced by D. Seebach and E.J. Corey. Polarity analysis during retrosynthetic analysis tells a chemist when umpolung tactics are required to synthesize a target molecule.

A cyclic compound is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where all the atoms are carbon, none of the atoms are carbon, or where both carbon and non-carbon atoms are present. Depending on the ring size, the bond order of the individual links between ring atoms, and their arrangements within the rings, carbocyclic and heterocyclic compounds may be aromatic or non-aromatic; in the latter case, they may vary from being fully saturated to having varying numbers of multiple bonds between the ring atoms. Because of the tremendous diversity allowed, in combination, by the valences of common atoms and their ability to form rings, the number of possible cyclic structures, even of small size numbers in the many billions.

In the analysis of the molecular formula of organic molecules, the degree of unsaturation (DU) (also known as the index of hydrogen deficiency (IHD), double bond equivalents (DBE), or unsaturation index) is a calculation that determines the total number of rings and π bonds. A formula is used in organic chemistry to help draw chemical structures. It does not give any information about those components individually—the specific number of rings, or of double bonds (one π bond each), or of triple bonds (two π bonds each). The final structure is verified with use of NMR, mass spectrometry and IR spectroscopy, as well as qualitative inspection. It is based on comparing the actual molecular formula to what would be a possible formula if the structure were saturated—having no rings and containing only σ bonds—with all atoms having their standard valence.

Morphinan is the prototype chemical structure of a large chemical class of psychoactive drugs, consisting of opiate analgesics, cough suppressants, and dissociative hallucinogens, among others. Typical examples include compounds such as morphine, codeine, and dextromethorphan (DXM). Despite related molecular structures, the pharmacological profiles and mechanisms of action between the various types of morphinan substances can vary substantially. They tend to function either as μ-opioid receptor agonists (analgesics), or NMDA receptor antagonists (dissociatives).
These drugs are known in the UK as controlled drug, because this is the term by which the act itself refers to them. In more general terms, however, many of these drugs are also controlled by the Medicines Act 1968, there are many other drugs which are controlled by the Medicines Act but not by the Misuse of Drugs Act, and some other drugs are controlled by other laws.

Stannole is an organotin compound with the formula (CH)4SnH2. It is classified as a metallole, i.e. an unsaturated five-membered ring containing a heteroatom. It is a structural analog of cyclopentadiene, with tin replacing the saturated carbon atom. Substituted derivatives, which have been synthesized, are also called stannoles.
1,3-Dioxane or m-dioxane is an organic compound with the molecular formula (CH2)4O2. It is a saturated six-membered heterocycle with two oxygen atoms in place of carbon atoms at the 1- and 3- positions. 1,4-Dioxane, which is of greater commercial value, is an isomer. Both dioxanes are colorless liquids.
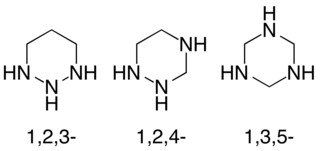
Triazinanes are a class of nitrogen-containing heterocycles. The parent molecules' molecular formula is (CH2)3(NH)3. They exist in three isomeric forms, 1,3,5-triazinanes being common. The triazinanes have six-membered cyclohexane-like ring but with three carbons replaced by nitrogens. Most commonly, the amines are tertiary.
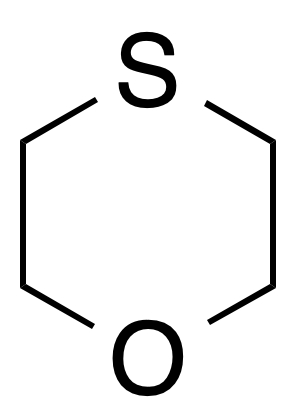
1,4-Oxathiane is a heterocyclic compound containing one oxygen atom and one sulfur atom at opposite corners of a saturated six-membered ring. By systematic numbering, the oxygen atom is position number 1, sulfur is number 4, and positions 2, 3, 5, and 6 are carbon atoms.












