Related Research Articles

Tadalafil, sold under the brand name Cialis among others, is a medication used to treat erectile dysfunction, benign prostatic hyperplasia, and pulmonary arterial hypertension. It is taken by mouth. Onset is typically within half an hour and the duration is up to 36 hours.
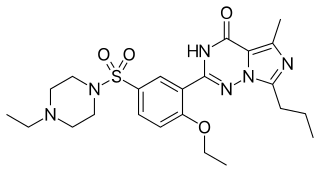
Vardenafil, sold under the brand name Levitra among others, is a medication that is used for treating erectile dysfunction. It is a PDE5 inhibitor. It is taken by mouth.

Finasteride, sold under the brand names Proscar and Propecia among others, is a medication used to treat pattern hair loss and benign prostatic hyperplasia (BPH) in men. It can also be used to treat excessive hair growth in women. It is usually taken orally but there are topical formulations for patients with hair loss, designed to minimize systemic exposure by acting specifically on hair follicles.
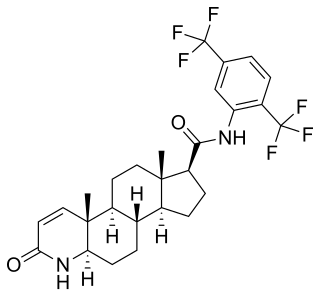
5α-Reductase inhibitors (5-ARIs), also known as dihydrotestosterone (DHT) blockers, are a class of medications with antiandrogenic effects which are used primarily in the treatment of enlarged prostate and scalp hair loss. They are also sometimes used to treat excess hair growth in women and as a component of hormone therapy for transgender women.

Dutasteride, sold under the brand name Avodart among others, is a medication primarily used to treat the symptoms of a benign prostatic hyperplasia (BPH), an enlarged prostate not associated with cancer. A few months may be required before benefits occur. It is also used for scalp hair loss in men and as a part of hormone therapy in transgender women. It is usually taken by mouth.

Alfuzosin, sold under the brand name Uroxatral among others, is a medication of the α1 blocker class. It is used to treat benign prostatic hyperplasia (BPH).
Alpha-1 blockers constitute a variety of drugs that block the effect of catecholamines on alpha-1-adrenergic receptors. They are mainly used to treat benign prostatic hyperplasia (BPH), hypertension and post-traumatic stress disorder. Alpha-1 adrenergic receptors are present in vascular smooth muscle, the central nervous system, and other tissues. When alpha blockers bind to these receptors in vascular smooth muscle, they cause vasodilation.
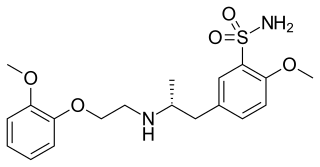
Tamsulosin, sold under the brand name Flomax among others, is a medication used to treat symptomatic benign prostatic hyperplasia (BPH) and chronic prostatitis and to help with the passage of kidney stones. The evidence for benefit with a kidney stone is better when the stone is larger. It is taken by mouth.
Chlordiazepoxide/clidinium bromide is a fixed-dose combination medication used to treat peptic ulcers, irritable bowel syndrome (IBS), and gastritis. It contains chlordiazepoxide and clidinium bromide. It helps relieve stomach spasms, abdominal cramps, and anxiety related to gastric disorders. Librax is a fixed ratio of these two medications and, as such, is not typically prescribed with an accompanying dosage, but rather directions on how many capsules to take per day. It comes in a capsule taken by mouth, usually three or four times daily, before meals and at bedtime. Chlordiazepoxide is an anti-anxiety medication belonging to the benzodiazepine class. Its use in IBS is thought to be due to its calming ability for patients that have IBS symptoms that are worsened by anxiety. Clidinium bromide is a synthetic quaternary ammonium antimuscarinic, a sub-class of a family of drugs known as anticholinergics. It treats IBS by decreasing gastrointestinal motility.

Cetrorelix, or cetrorelix acetate, sold under the brand name Cetrotide, is an injectable gonadotropin-releasing hormone (GnRH) antagonist. A synthetic decapeptide, it is used in assisted reproduction to inhibit premature luteinizing hormone surges The drug works by blocking the action of GnRH upon the pituitary, thus rapidly suppressing the production and action of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). In addition, cetrorelix can be used to treat hormone-sensitive cancers of the prostate and breast and some benign gynaecological disorders. It is administered as either multiple 0.25 mg daily subcutaneous injections or as a single-dose 3 mg subcutaneous injection. The duration of the 3 mg single dose is four days; if human chorionic gonadotropin (hCG) is not administered within four days, a daily 0.25 mg dose is started and continued until hCG is administered.

Silodosin, sold under the brand name Urief among others, is a medication for the symptomatic treatment of benign prostatic hyperplasia. It acts as an alpha-1 adrenergic receptor antagonist.

Rilpivirine, sold under the brand names Edurant and Rekambys, is a medication, developed by Tibotec, used for the treatment of HIV/AIDS. It is a second-generation non-nucleoside reverse transcriptase inhibitor (NNRTI) with higher potency, longer half-life and reduced side-effect profile compared with older NNRTIs such as efavirenz.

Naltrexone/bupropion, sold under the brand name Contrave among others, is a fixed-dose combination medication for the management of chronic obesity in adults in combination with a reduced-calorie diet and increased physical activity. It contains naltrexone, an opioid antagonist, and bupropion, an aminoketone antidepressant. It is taken by mouth. Both medications have individually shown some evidence of effectiveness in weight loss, and the combination has been shown to have some synergistic effects on weight.
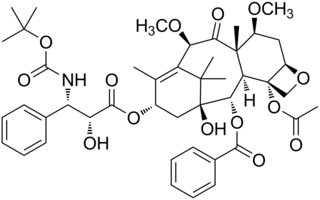
Cabazitaxel, sold under the brand name Jevtana, is a semi-synthetic derivative of a natural taxoid. It is a microtubule inhibitor, and the fourth taxane to be approved as a cancer therapy.
Netupitant/palonosetron, sold under the brand name Akynzeo, is a fixed-dose combination medication used for the prevention of acute and delayed chemotherapy-induced nausea and vomiting. It is marketed and distributed by Helsinn Therapeutics. Netupitant is an NK1 receptor antagonist and palonosetron is a 5-HT3 receptor antagonist.

Glasdegib, sold under the brand name Daurismo, is a medication for the treatment of newly-diagnosed acute myeloid leukemia (AML) in adults older than 75 years or those who have comorbidities that preclude use of intensive induction chemotherapy. It is taken by mouth and is used in combination with low-dose cytarabine.
Empagliflozin/metformin, sold under the brand name Synjardy among others, is a fixed-dose combination anti-diabetic medication used to treat type 2 diabetes. It contains empagliflozin and metformin hydrochloride. It is taken by mouth.
Dapagliflozin/metformin, sold under the brand name Xigduo XR amongst others, is a fixed-dose combination anti-diabetic medication used as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes. It is a combination of dapagliflozin and metformin and is taken by mouth.. Dapagliflozin/metformin was approved for use in the European Union in January 2014, in the United States in February 2014, and in Australia in July 2014.
Canagliflozin/metformin, sold under the brand name Vokanamet among others, is a fixed-dose combination anti-diabetic medication used for the treatment of type 2 diabetes. It is used in combination with diet and exercise. It is taken by mouth.
Finasteride/tadalafil, sold under the brand name Entadfi, is a fixed-dose combination medication used for the treatment of benign prostatic hyperplasia (BPH). It contains finasteride and tadalafil. It is taken by mouth.
References
- 1 2 "Jalyn - dutasteride and tamsulosin hydrochloride capsule". DailyMed. U.S. National Library of Medicine. Retrieved 28 December 2020.
- ↑ Keating GM (May 2012). "Dutasteride/tamsulosin: in benign prostatic hyperplasia". Drugs & Aging. 29 (5): 405–19. doi:10.2165/11208920-000000000-00000. PMID 22550968. S2CID 209142875.
- ↑ "Approval Package for: Dutasteride 0.5 mg/Tamsulosin hydrochloride 0.4 mg" (PDF). Center for Drug Evaluation and Research. Food and Drug Administration. 14 June 2010.
- ↑ "Detailed View: Safety Labeling Changes: Jalyn (dutasteride and tamsulosin) capsules". Center for Drug Evaluation and Research. U.S. Food and Drug Administration. June 2011. Archived from the original on 6 January 2012.