In chemistry, an alcohol is any organic compound in which the hydroxyl functional group (–OH) is bound to a carbon. The term alcohol originally referred to the primary alcohol ethanol, which is used as a drug and is the main alcohol present in alcoholic beverages. An important class of alcohols, of which methanol and ethanol are the simplest members, includes all compounds for which the general formula is CnH2n+1OH. It is these simple monoalcohols that are the subject of this article.

An aldehyde is an organic compound containing a functional group with the structure −CHO, consisting of a carbonyl center with the carbon atom also bonded to hydrogen and to an R group, which is any generic alkyl or side chain. The group—without R—is the aldehyde group, also known as the formyl group. Aldehydes are common in organic chemistry, and many fragrances are aldehydes.
Acetophenone is the organic compound with the formula C6H5C(O)CH3 (also represented by the pseudoelement symbols PhAc or BzMe). It is the simplest aromatic ketone. This colorless, viscous liquid is a precursor to useful resins and fragrances.
Acetaldehyde (systematic name ethanal) is an organic chemical compound with the formula CH3CHO, sometimes abbreviated by chemists as MeCHO (Me = methyl). It is one of the most important aldehydes, occurring widely in nature and being produced on a large scale in industry. Acetaldehyde occurs naturally in coffee, bread, and ripe fruit, and is produced by plants. It is also produced by the partial oxidation of ethanol by the liver enzyme alcohol dehydrogenase and is a contributing cause of hangover after alcohol consumption. Pathways of exposure include air, water, land, or groundwater, as well as drink and smoke. Consumption of disulfiram inhibits acetaldehyde dehydrogenase, the enzyme responsible for the metabolism of acetaldehyde, thereby causing it to build up in the body.

Ethylene oxide, called oxirane by IUPAC, is an organic compound with the formula C
2H
4O. It is a cyclic ether and the simplest epoxide: a three-membered ring consisting of one oxygen atom and two carbon atoms. Ethylene oxide is a colorless and flammable gas with a faintly sweet odor. Because it is a strained ring, ethylene oxide easily participates in a number of addition reactions that result in ring-opening. Ethylene oxide is isomeric with acetaldehyde and with vinyl alcohol. Ethylene oxide is industrially produced by oxidation of ethylene in the presence of silver catalyst.
A diol is a chemical compound containing two hydroxyl groups. An aliphatic diol is also called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified.
Dehydrogenation is a chemical reaction that involves the removal of hydrogen from an organic molecule.It is the reverse of hydrogenation. Dehydrogenation is an important reaction because it converts alkanes, which are relatively inert and thus low-valued, to olefins, which are reactive and thus more valuable. Alkenes are precursors to aldehydes, alcohols, polymers, and aromatics. Dehydrogenation processes are used extensively to produce aromatics and styrene in the petrochemical industry. Such processes are highly endothermic and require temperatures of 500 °C and above. Dehydrogenation also converts saturated fats to unsaturated fats. Enzymes that catalyze dehydrogenation are called dehydrogenases.
Steam reforming or steam methane reforming is a chemical synthesis for producing syngas, hydrogen, carbon monoxide from hydrocarbon fuels such as natural gas. This is achieved in a processing device called a reformer which reacts steam at high temperature and pressure with methane in the presence of a nickel catalyst. The steam methane reformer is widely used in industry to make hydrogen, also called "blue hydrogen", from natural gas. With the use of CCUS technology it is possible to capture the carbon dioxide in the process and thus decarbonised natural gas.
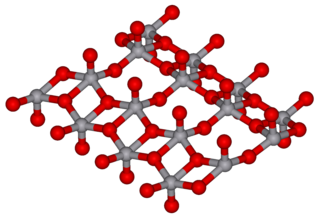
Vanadium(V) oxide (vanadia) is the inorganic compound with the formula V2O5. Commonly known as vanadium pentoxide, it is a brown/yellow solid, although when freshly precipitated from aqueous solution, its colour is deep orange. Because of its high oxidation state, it is both an amphoteric oxide and an oxidizing agent. From the industrial perspective, it is the most important compound of vanadium, being the principal precursor to alloys of vanadium and is a widely used industrial catalyst.

Methyl methacrylate (MMA) is an organic compound with the formula CH2=C(CH3)COOCH3. This colorless liquid, the methyl ester of methacrylic acid (MAA) is a monomer produced on a large scale for the production of poly(methyl methacrylate) (PMMA).

A carbonate ester (organic carbonate or organocarbonate) is an ester of carbonic acid. This functional group consists of a carbonyl group flanked by two alkoxy groups. The general structure of these carbonates is R1O(C=O)OR2 and they are related to esters R1O(C=O)R and ethers R1OR2 and also to the inorganic carbonates.
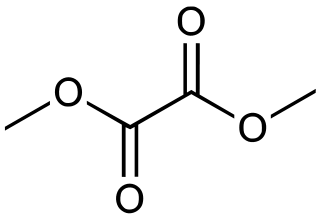
Dimethyl oxalate is the organic compound with the formula (CH3O2C)2. It is the dimethyl ester of oxalic acid. Dimethyl oxalate is a colorless or white solid that is soluble in water.
Carbonylation refers to reactions that introduce carbon monoxide into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemistry. The term carbonylation also refers to oxidation of protein side chains.

Acetic acid, systematically named ethanoic acid, is a colourless liquid organic compound with the chemical formula CH3COOH (also written as CH3CO2H or C2H4O2). When undiluted, it is sometimes called glacial acetic acid. Vinegar is no less than 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water. Acetic acid has a distinctive sour taste and pungent smell. In addition to household vinegar, it is mainly produced as a precursor to polyvinyl acetate and cellulose acetate. It is classified as a weak acid since it only partially dissociates in solution, but concentrated acetic acid is corrosive and can attack the skin.
Catalytic oxidation are processes that oxidize compounds using catalysts. Common applications involve oxidation of organic compounds by the oxygen in air. Such processes are conducted on a large scale for the remediation of pollutants, production of valuable chemicals, and the production of energy.
In chemistry, ammoxidation is an industrial process for the production of nitriles using ammonia and oxygen. The usual substrates are alkenes. It is sometimes called the Sohio process, acknowledging that ammoxidation was first discovered by Standard Oil of Ohio in 1957. An important application of this process is the production of acrylonitrile:
The first time a catalyst was used in the industry was in 1746 by J. Hughes in the manufacture of lead chamber sulfuric acid. Since then catalysts have been in use in a large portion of the chemical industry. In the start only pure components were used as catalysts, but after the year 1900 multicomponent catalysts were studied and are now commonly used in the industry.
Methane functionalization is the process of converting methane in its gaseous state to another molecule with a functional group, typically methanol or acetic acid, through the use of transition metal catalysts.