In chemistry, a carbide usually describes a compound composed of carbon and a metal. In metallurgy, carbiding or carburizing is the process for producing carbide coatings on a metal piece.
The lanthanide or lanthanoid series of chemical elements comprises at least the 14 metallic chemical elements with atomic numbers 57–70, from lanthanum through ytterbium. In the periodic table, they fill the 4f orbitals. Lutetium is also sometimes considered a lanthanide, despite being a d-block element and a transition metal.

In crystallography, the cubiccrystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.

An intermetallic is a type of metallic alloy that forms an ordered solid-state compound between two or more metallic elements. Intermetallics are generally hard and brittle, with good high-temperature mechanical properties. They can be classified as stoichiometric or nonstoichiometic intermetallic compounds.
A boride is a compound between boron and a less electronegative element, for example silicon boride (SiB3 and SiB6). The borides are a very large group of compounds that are generally high melting and are covalent more than ionic in nature. Some borides exhibit very useful physical properties. The term boride is also loosely applied to compounds such as B12As2 (N.B. Arsenic has an electronegativity higher than boron) that is often referred to as icosahedral boride.
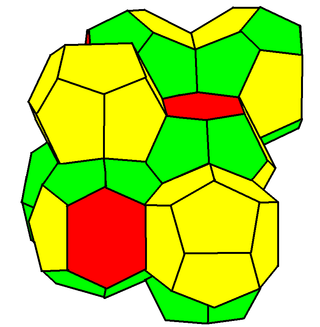
In geometry, the Weaire–Phelan structure is a three-dimensional structure representing an idealised foam of equal-sized bubbles, with two different shapes. In 1993, Denis Weaire and Robert Phelan found that this structure was a better solution of the Kelvin problem of tiling space by equal volume cells of minimum surface area than the previous best-known solution, the Kelvin structure.
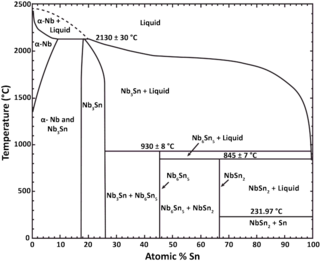
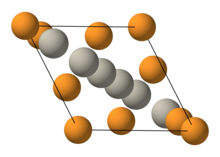
Niobium–tin is an intermetallic compound of niobium (Nb) and tin (Sn), used industrially as a type-II superconductor. This intermetallic compound has a simple structure: A3B. It is more expensive than niobium–titanium (NbTi), but remains superconducting up to a magnetic flux density of 30 teslas [T] (300,000 G), compared to a limit of roughly 15 T for NbTi.

The A15 phases (also known as β-W or Cr3Si structure types) are series of intermetallic compounds with the chemical formula A3B (where A is a transition metal and B can be any element) and a specific structure. The A15 phase is also one of the members in the Frank–Kasper phases family. Many of these compounds have superconductivity at around 20 K (−253 °C; −424 °F), which is comparatively high, and remain superconductive in magnetic fields of tens of teslas (hundreds of kilogauss). This kind of superconductivity (Type-II superconductivity) is an important area of study as it has several practical applications.
Magnesium aluminide is an intermetallic compound of magnesium and aluminium. Common phases (molecular structures) include the beta phase (Mg2Al3) and the gamma phase (Mg17Al12), which both have cubic crystal structures. Magnesium aluminides are important constituents of 5XXX aluminium alloys (aluminium-magnesium) and magnesium-aluminium alloys, determining many of their engineering properties. Due to the advantage of low density and being strong, magnesium aluminide is important for aircraft engines. MgAl has also been investigated for use as a reactant to produce metal hydrides in hydrogen storage technology. Like many intermetallics, MgAl compounds often have unusual stoichiometries with large and complex unit cells.

Non-stoichiometric compounds are chemical compounds, almost always solid inorganic compounds, having elemental composition whose proportions cannot be represented by a ratio of small natural numbers ; most often, in such materials, some small percentage of atoms are missing or too many atoms are packed into an otherwise perfect lattice work.
Complex metallic alloys (CMAs) or complex intermetallics (CIMs) are intermetallic compounds characterized by the following structural features:
- large unit cells, comprising some tens up to thousands of atoms,
- the presence of well-defined atom clusters, frequently of icosahedral point group symmetry,
- the occurrence of inherent disorder in the ideal structure.
In chemistry, a Zintl phase is a product of a reaction between a group 1 or group 2 and main group metal or metalloid. It is characterized by intermediate metallic/ionic bonding. Zintl phases are a subgroup of brittle, high-melting intermetallic compounds that are diamagnetic or exhibit temperature-independent paramagnetism and are poor conductors or semiconductors.
A crystallographic database is a database specifically designed to store information about the structure of molecules and crystals. Crystals are solids having, in all three dimensions of space, a regularly repeating arrangement of atoms, ions, or molecules. They are characterized by symmetry, morphology, and directionally dependent physical properties. A crystal structure describes the arrangement of atoms, ions, or molecules in a crystal.

Copper(I) sulfide is a copper sulfide, a chemical compound of copper and sulfur. It has the chemical compound Cu2S. It is found in nature as the mineral chalcocite. It has a narrow range of stoichiometry ranging from Cu1.997S to Cu2.000S. Samples are typically black.

A chemical compound is a chemical substance composed of many identical molecules containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken and/or new bonds formed.
A stannide can refer to an intermetallic compound containing tin combined with one or more other metals; an anion consisting solely of tin atoms or a compound containing such an anion, or, in the field of organometallic chemistry an ionic compound containing an organotin anion
Metals, and specifically rare-earth elements, form numerous chemical complexes with boron. Their crystal structure and chemical bonding depend strongly on the metal element M and on its atomic ratio to boron. When B/M ratio exceeds 12, boron atoms form B12 icosahedra which are linked into a three-dimensional boron framework, and the metal atoms reside in the voids of this framework. Those icosahedra are basic structural units of most allotropes of boron and boron-rich rare-earth borides. In such borides, metal atoms donate electrons to the boron polyhedra, and thus these compounds are regarded as electron-deficient solids.
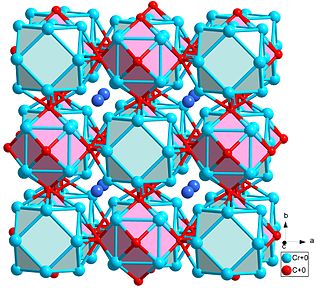
Cr23C6 is the prototypical compound of a common crystal structure, discovered in 1933 as part of the chromium-carbon binary phase diagram. Over 85 known compounds adopt this structure type, which can be described as a NaCl-like packing of chromium cubes and cuboctahedra.
In crystallography, a Strukturbericht designation or Strukturbericht type is a system of detailed crystal structure classification by analogy to another known structure. The designations were intended to be comprehensive but are mainly used as supplement to space group crystal structures designations, especially historically. Each Strukturbericht designation is described by a single space group, but the designation includes additional information about the positions of the individual atoms, rather than just the symmetry of the crystal structure. While Strukturbericht symbols exist for many of the earliest observed and most common crystal structures, the system is not comprehensive, and is no longer being updated. Modern databases such as Inorganic Crystal Structure Database index thousands of structure types directly by the prototype compound. These are essentially equivalent to the old Stukturbericht designations.

UPt3 is an inorganic binary intermetallic crystalline compound of platinum and uranium.