A carbon–carbon bond is a covalent bond between two carbon atoms. The most common form is the single bond: a bond composed of two electrons, one from each of the two atoms. The carbon–carbon single bond is a sigma bond and is formed between one hybridized orbital from each of the carbon atoms. In ethane, the orbitals are sp3-hybridized orbitals, but single bonds formed between carbon atoms with other hybridizations do occur. In fact, the carbon atoms in the single bond need not be of the same hybridization. Carbon atoms can also form double bonds in compounds called alkenes or triple bonds in compounds called alkynes. A double bond is formed with an sp2-hybridized orbital and a p-orbital that is not involved in the hybridization. A triple bond is formed with an sp-hybridized orbital and two p-orbitals from each atom. The use of the p-orbitals forms a pi bond.
In organic chemistry, a carbanion is an anion in which carbon is trivalent and bears a formal negative charge.

Triphenylmethane, or triphenyl methane, is the hydrocarbon with the formula (C6H5)3CH. This colorless solid is soluble in nonpolar organic solvents and not in water. Triphenylmethane is the basic skeleton of many synthetic dyes called triarylmethane dyes, many of them are pH indicators, and some display fluorescence. A trityl group in organic chemistry is a triphenylmethyl group Ph3C, e.g. triphenylmethyl chloride (trityl chloride) and the triphenylmethyl radical (trityl radical).
In organometallic chemistry, acetylide refers to chemical compounds with the chemical formulas MC≡CH and MC≡CM, where M is a metal. The term is used loosely and can refer to substituted acetylides having the general structure RC−CM. Acetylides are reagents in organic synthesis. The calcium acetylide commonly called calcium carbide is a major compound of commerce.

Moses Gomberg was a chemistry professor at the University of Michigan. He was elected to the National Academy of Sciences and served as president of the American Chemical Society.

Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group [R−N+≡N]X− where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halide.

Phenyllithium or lithobenzene is an organometallic agent with the empirical formula C6H5Li. It is most commonly used as a metalating agent in organic syntheses and a substitute for Grignard reagents for introducing phenyl groups in organic syntheses. Crystalline phenyllithium is colorless; however, solutions of phenyllithium are various shades of brown or red depending on the solvent used and the impurities present in the solute.
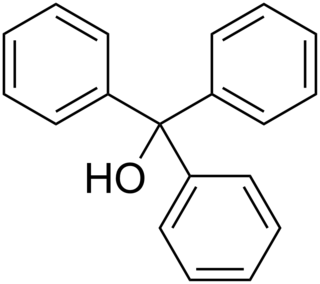
Triphenylmethanol is an organic compound. It is a white crystalline solid that is insoluble in water and petroleum ether, but well soluble in ethanol, diethyl ether, and benzene. In strongly acidic solutions, it produces an intensely yellow color, due to the formation of a stable "trityl" carbocation. Many derivatives of triphenylmethanol are important dyes.

The triphenylmethyl radical (often shorted to trityl radical) is an organic compound with the formula (C6H5)3C. It is a persistent radical. It was the first radical ever to be described in organic chemistry. Because of its accessibility, the trityl radical has been heavily exploited.

Tetraphenylmethane is an organic compound consisting of a methane core with four phenyl substituents. It was first synthesized by Moses Gomberg in 1898.

Hexaphenylethane is a hypothetical organic compound consisting of an ethane core with six phenyl substituents. All attempts at its synthesis have been unsuccessful. The trityl free radical, Ph3C ·, was originally thought to dimerize to form hexaphenylethane. However, an inspection of the NMR spectrum of this dimer reveals that it is in fact a non-symmetrical species, Gomberg's dimer instead.

Fluoroboric acid or tetrafluoroboric acid (archaically, fluoboric acid) is an inorganic compound with the chemical formula [H+][BF4−], where H+ represents the solvated proton. The solvent can be any suitably Lewis-basic entity. For instance, in water, it can be represented by H
3OBF
4 (oxonium tetrafluoroborate), although more realistically, several water molecules solvate the proton: [H(H2O)n+][BF4−]. The ethyl ether solvate is also commercially available: [H(Et2O)n+][BF4−], where n is most likely 2. Unlike other strong acids like H2SO4 or HClO4, the pure unsolvated substance does not exist (see below).

Triphenylmethyl chloride or trityl chloride (TrCl) is a white solid with the chemical formula C19H15Cl. It is an alkyl halide, sometimes used to introduce the trityl protecting group.
Organosodium chemistry is the chemistry of organometallic compounds containing a carbon to sodium chemical bond. The application of organosodium compounds in chemistry is limited in part due to competition from organolithium compounds, which are commercially available and exhibit more convenient reactivity.
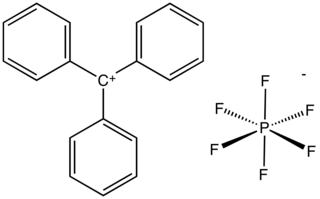
Triphenylmethylhexafluorophosphate is an organic salt with the formula C
19H
15F
6P or (C
6H
5)
3CPF
6, consisting of the triphenylmethyl cation [(C
6H
5)
3C]+
and the hexafluorophosphate anion [PF
6]−
.
Radical theory is an obsolete scientific theory in chemistry describing the structure of organic compounds. The theory was pioneered by Justus von Liebig, Friedrich Wöhler and Auguste Laurent around 1830 and is not related to the modern understanding of free radicals. In this theory, organic compounds were thought to exist as combinations of radicals that could be exchanged in chemical reactions just as chemical elements could be interchanged in inorganic compounds.

Bis(cyclopentadienyl)titanium(III) chloride, also known as the Nugent–RajanBabu reagent, is the organotitanium compound which exists as a dimer with the formula [(C5H5)2TiCl]2. It is an air sensitive green solid. The complex finds specialized use in synthetic organic chemistry as a single electron reductant.
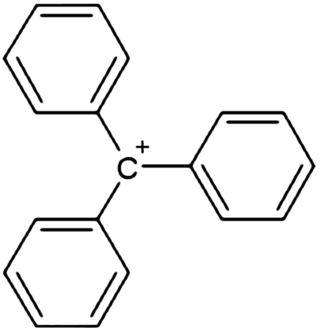
In chemistry, triphenylcarbenium, triphenylmethyl cation, tritylium , or trityl cation is an ion with formula [C
19H
15]+
or (C
6H
5)
3C+
, consisting of a carbon atom with a positive charge connected to three phenyl groups. It is a charged version of the triphenylmethyl radical (C
6H
5)
3C•. The name is often abbreviated to triphenylmethyl or trityl in salts, although these names also denote the chemical group in compounds like triphenylmethyl chloride that do not contain the cation.

A trivalent group 14 radical (also known as a trivalent tetrel radical) is a molecule that contains a group 14 element (E = C, Si, Ge, Sn, Pb) with three bonds and a free radical, having the general formula of R3E•. Such compounds can be categorized into three different types, depending on the structure (or equivalently the orbital in which the unpaired electron resides) and the energetic barrier to inversion. A molecule that remains rigidly in a pyramidal structure has an electron in a sp3 orbital is denoted as Type A. A structure that is pyramidal, but flexible, is denoted as Type B. And a planar structure with an electron that typically would reside in a pure p orbital is denoted as Type C. The structure of such molecules has been determined by probing the nature of the orbital that the unpaired electron resides in using spectroscopy, as well as directly with X-ray methods. Trivalent tetrel radicals tend to be synthesized from their tetravalent counterparts (i.e. R3EY where Y is a species that will dissociate).
Dispersion stabilized molecules are molecules where the London dispersion force (LDF), a non-covalent attractive force between atoms and molecules, plays a significant role in promoting the molecule's stability. Distinct from steric hindrance, dispersion stabilization has only recently been considered in depth by organic and inorganic chemists after earlier gaining prominence in protein science and supramolecular chemistry. Although usually weaker than covalent bonding and other forms of non-covalent interactions like hydrogen bonding, dispersion forces are known to be a significant if not dominating stabilizing force in certain organic, inorganic, and main group molecules, stabilizing otherwise reactive moieties and exotic bonding.
















