Digital Imaging and Communications in Medicine (DICOM) is a technical standard for the digital storage and transmission of medical images and related information. It includes a file format definition, which specifies the structure of a DICOM file, as well as a network communication protocol that uses TCP/IP to communicate between systems. The primary purpose of the standard is to facilitate communication between the software and hardware entities involved in medical imaging, especially those that are created by different manufacturers. Entities that utilize DICOM files include components of picture archiving and communication systems (PACS), such as imaging machines (modalities), radiological information systems (RIS), scanners, printers, computing servers, and networking hardware.
Health Level Seven or HL7 is a range of global standards for the transfer of clinical and administrative health data between applications. The HL7 standards focus on the application layer, which is "layer 7" in the Open Systems Interconnection model. The standards are produced by Health Level Seven International, an international standards organization, and are adopted by other standards issuing bodies such as American National Standards Institute and International Organization for Standardization. There are a range of primary standards that are commonly used across the industry, as well as secondary standards which are less frequently adopted.
An electronic health record (EHR) is the systematized collection of patient and population electronically stored health information in a digital format. These records can be shared across different health care settings. Records are shared through network-connected, enterprise-wide information systems or other information networks and exchanges. EHRs may include a range of data, including demographics, medical history, medication and allergies, immunization status, laboratory test results, radiology images, vital signs, personal statistics like age and weight, and billing information.

A medical device is any device intended to be used for medical purposes. Significant potential for hazards are inherent when using a device for medical purposes and thus medical devices must be proved safe and effective with reasonable assurance before regulating governments allow marketing of the device in their country. As a general rule, as the associated risk of the device increases the amount of testing required to establish safety and efficacy also increases. Further, as associated risk increases the potential benefit to the patient must also increase.
IEC 61850 is an international standard defining communication protocols for intelligent electronic devices at electrical substations. It is a part of the International Electrotechnical Commission's (IEC) Technical Committee 57 reference architecture for electric power systems. The abstract data models defined in IEC 61850 can be mapped to a number of protocols. Current mappings in the standard are to Manufacturing Message Specification (MMS), GOOSE [see section 3, Terms and definitions, term 3.65 on page 14], SV or SMV, and soon to web services. In the previous version of the standard, GOOSE stood for "Generic Object Oriented Substation Event", but this old definition is still very common in IEC 61850 documentation. These protocols can run over TCP/IP networks or substation LANs using high speed switched Ethernet to obtain the necessary response times below four milliseconds for protective relaying.
openEHR is an open standard specification in health informatics that describes the management and storage, retrieval and exchange of health data in electronic health records (EHRs). In openEHR, all health data for a person is stored in a "one lifetime", vendor-independent, person-centred EHR. The openEHR specifications include an EHR Extract specification but are otherwise not primarily concerned with the exchange of data between EHR-systems as this is the focus of other standards such as EN 13606 and HL7.

SNOMED CT or SNOMED Clinical Terms is a systematically organized computer-processable collection of medical terms providing codes, terms, synonyms and definitions used in clinical documentation and reporting. SNOMED CT is considered to be the most comprehensive, multilingual clinical healthcare terminology in the world. The primary purpose of SNOMED CT is to encode the meanings that are used in health information and to support the effective clinical recording of data with the aim of improving patient care. SNOMED CT provides the core general terminology for electronic health records. SNOMED CT comprehensive coverage includes: clinical findings, symptoms, diagnoses, procedures, body structures, organisms and other etiologies, substances, pharmaceuticals, devices and specimens.

Continua Health Alliance is an international non-profit, open industry group of nearly 240 healthcare providers, communications, medical, and fitness device companies. Continua was a founding member of Personal Connected Health Alliance which was launched in February 2014 with other founding members mHealth SUMMIT and HIMSS.
The European Committee for Standardization (CEN) Standard Architecture for Healthcare Information Systems, Health Informatics Service Architecture or HISA is a standard that provides guidance on the development of modular open information technology (IT) systems in the healthcare sector. Broadly, architecture standards outline frameworks which can be used in the development of consistent, coherent applications, databases and workstations. This is done through the definition of hardware and software construction requirements and outlining of protocols for communications. The HISA standard provides a formal standard for a service-oriented architecture (SOA), specific for the requirements of health services, based on the principles of Open Distributed Processing. The HISA standard evolved from previous work on healthcare information systems architecture commenced by Reseau d’Information et de Communication Hospitalier Europeen (RICHE) in 1989, and subsequently built upon by a number of organizations across Europe.
Medical equipment management is a term for the professionals who manage operations, analyze and improve utilization and safety, and support servicing healthcare technology. These healthcare technology managers are, much like other healthcare professionals referred to by various specialty or organizational hierarchy names.
The system of concepts to support continuity of care, often referred to as ContSys, is an ISO and CEN standard . Continuity of care is an organisational principle that represents an important aspect of quality and safety in health care. Semantic interoperability is a basic requirement for continuity of care. Concepts that are needed for these purposes must represent both the content and context of the health care services.

In medicine, monitoring is the observation of a disease, condition or one or several medical parameters over time.
Barcode technology in healthcare is the use of optical machine-readable representation of data in a hospital or healthcare setting.

A body area network (BAN), also referred to as a wireless body area network (WBAN) or a body sensor network (BSN) or a medical body area network (MBAN), is a wireless network of wearable computing devices. BAN devices may be embedded inside the body as implants or pills, may be surface-mounted on the body in a fixed position, or may be accompanied devices which humans can carry in different positions, such as in clothes pockets, by hand, or in various bags. Devices are becoming smaller, especially in body area networks. These networks include multiple small body sensor units (BSUs) and a single central unit (BCU). Despite this trend, decimeter sized smart devices still play an important role. They act as data hubs or gateways and provide a user interface for viewing and managing BAN applications on the spot. The development of WBAN technology started around 1995 around the idea of using wireless personal area network (WPAN) technologies to implement communications on, near, and around the human body. About six years later, the term "BAN" came to refer to systems where communication is entirely within, on, and in the immediate proximity of a human body. A WBAN system can use WPAN wireless technologies as gateways to reach longer ranges. Through gateway devices, it is possible to connect the wearable devices on the human body to the internet. This way, medical professionals can access patient data online using the internet independent of the patient location.
ISO/IEEE 11073 Personal Health Device (PHD) standards are a group of standards addressing the interoperability of personal health devices (PHDs) such as weighing scales, blood pressure monitors, blood glucose monitors and the like. The standards draw upon earlier IEEE11073 standards work, but differ from this earlier work due to an emphasis on devices for personal use and a simpler communications model.
The Open Smart Grid Protocol (OSGP) is a family of specifications published by the European Telecommunications Standards Institute (ETSI) used in conjunction with the ISO/IEC 14908 control networking standard for smart grid applications. OSGP is optimized to provide reliable and efficient delivery of command and control information for smart meters, direct load control modules, solar panels, gateways, and other smart grid devices. With over 5 million OSGP based smart meters and devices deployed worldwide it is one of the most widely used smart meter and smart grid device networking standards.
Medical device connectivity is the establishment and maintenance of a connection through which data is transferred between a medical device, such as a patient monitor, and an information system. The term is used interchangeably with biomedical device connectivity or biomedical device integration. By eliminating the need for manual data entry, potential benefits include faster and more frequent data updates, diminished human error, and improved workflow efficiency.

Dipak Kalra is President of the European Institute for Health Records and of the European Institute for Innovation through Health Data. He undertakes international research and standards development, and advises on adoption strategies, relating to Electronic Health Records.

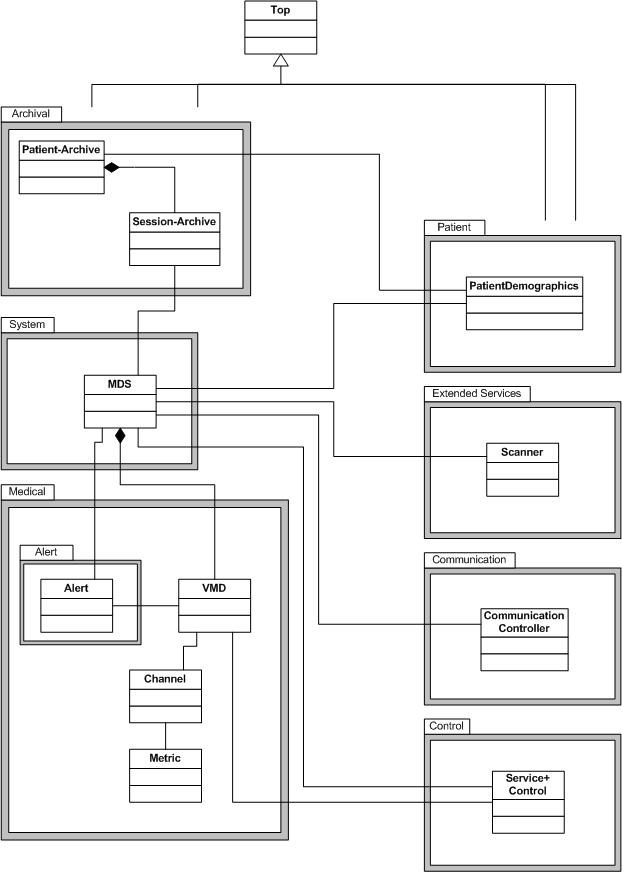
The IEEE 11073 service-oriented device connectivity (SDC) family of standards defines a communication protocol for point-of-care (PoC) medical devices. The main purpose is to enable manufacturer-independent medical device-to-device interoperability. Furthermore, interconnection between medical devices and medical information systems is enabled. However, IEEE 11073 SDC does not compete with established and emerging standards like HL7 v2 or HL7 FHIR. IEEE 11073 SDC is part of the established ISO/IEEE 11073 family of standards.
Clinical data standards are used to store and communicate information related to healthcare so that its meaning is unambiguous. They are used in clinical practice, in activity analysis and finding, and in research and development.