External links
| International | |
|---|---|
| National | |
| | This pharmacology-related article is a stub. You can help Wikipedia by expanding it. |
| | This article about an organisation based in Italy is a stub. You can help Wikipedia by expanding it. |
The Italian Medicines Agency (Agenzia italiana del farmaco, AIFA) is the public institution responsible for the regulatory activity of pharmaceuticals in Italy.

The University of Turin is a public research university in the city of Turin, in the Piedmont region of Italy. It is one of the oldest universities in Europe and continues to play an important role in research and training. It is steadily ranked among the top 5 Italian universities and it is ranked third for research activities in Italy, according to the latest data by ANVUR.

The European Medicines Agency (EMA) is an agency of the European Union (EU) in charge of the evaluation and supervision of pharmaceutical products. Prior to 2004, it was known as the European Agency for the Evaluation of Medicinal Products or European Medicines Evaluation Agency (EMEA).

Nimesulide is a nonsteroidal anti-inflammatory drug (NSAID) with pain medication and fever reducing properties. Its approved indications are the treatment of acute pain, the symptomatic treatment of osteoarthritis, and primary dysmenorrhoea in adolescents and adults above 12 years old.

Doxorubicin, sold under the brand name Adriamycin among others, is a chemotherapy medication used to treat cancer. This includes breast cancer, bladder cancer, Kaposi's sarcoma, lymphoma, and acute lymphocytic leukemia. It is often used together with other chemotherapy agents. Doxorubicin is given by injection into a vein.
Marianne Merchez is a Belgian doctor from the Catholic University of Louvain and a former European Space Agency astronaut. She is certified in aerospace medicine and in industrial medicine, and she is also a professional pilot.

Narcobarbital (Pronarcon) is a barbiturate derivative developed in 1932 by Carl Heinrich Friedrich Boedecker and Heinrich Gruber Schoneberg, assignors to the firm J. D. Riedel-E. de Haën AG, Berlin, Germany. Later, in 1937, may, was patented in United States. It is an N-methylated derivative of propallylonal and has similar sedative effects. It is still used in veterinary medicine for inducing surgical anaesthesia.
The Committee for Medicinal Products for Human Use (CHMP), formerly known as the Committee for Proprietary Medicinal Products (CPMP), is the European Medicines Agency's committee responsible for elaborating the agency's opinions on all issues regarding medicinal products for human use.

Norfenfluramine, or 3-trifluoromethylamphetamine, is a never-marketed drug of the amphetamine family that behaves as a serotonin and norepinephrine releasing agent and potent 5-HT2A, 5-HT2B, and 5-HT2C agonist. The action of norfenfluramine on 5-HT2B receptors on heart valves leads to a characteristic pattern of heart failure following proliferation of cardiac fibroblasts on the tricuspid valve, known as cardiac fibrosis. This side effect led to the withdrawal of fenfluramine as an anorectic agent worldwide, and to the withdrawal of benfluorex in Europe, as both fenfluramine and benfluorex form norfenfluramine as an active metabolite. It is a human TAAR1 agonist.

The Bureau of Medicine and Surgery (BUMED) is an agency of the United States Department of the Navy that manages health care activities for the United States Navy and the United States Marine Corps. BUMED operates hospitals and other healthcare facilities as well as laboratories for biomedical research, and trains and manages the Navy's many staff corps related to medicine. Its headquarters is located at the Defense Health Headquarters in Fairfax County, Virginia. BUMED has 41,930 medical personnel and more than a million eligible beneficiaries.
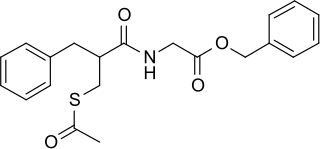
Racecadotril, also known as acetorphan, is an antidiarrheal medication which acts as a peripheral enkephalinase inhibitor. Unlike other opioid medications used to treat diarrhea, which reduce intestinal motility, racecadotril has an antisecretory effect — it reduces the secretion of water and electrolytes into the intestine. It is available in France and other European countries as well as most of South America and some South East Asian countries, but not in the United States. It is sold under the tradename Hidrasec, among others. Thiorphan is the active metabolite of racecadotril, which exerts the bulk of its inhibitory actions on enkephalinases.
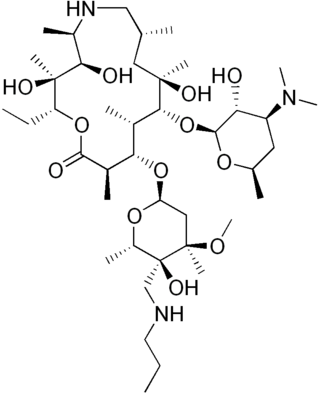
Tulathromycin, sold under the brand name Draxxin among others, is a macrolide antibiotic used to treat bovine respiratory disease in cattle and swine respiratory disease in pigs.

Firocoxib, sold under the brand names Equioxx and Previcox among others, is a nonsteroidal anti-inflammatory drug of the COX-2 inhibitor (coxib) class, approved for use in horses (Equioxx) and for use in dogs (Previcox). Firocoxib was the first COX-2 inhibitor approved by the U.S. Food and Drug Administration for horses. Firocoxib is not intended or approved for use in human medicine.

Flupirtine is an aminopyridine that functions as a centrally acting non-opioid analgesic that was originally used as an analgesic for acute and chronic pain but in 2013 due to issues with liver toxicity, the European Medicines Agency restricted its use to acute pain, for no more than two weeks, and only for people who cannot use other painkillers. In March 2018, marketing authorisations for flupirtine were withdrawn following a European Medicines Agency recommendation based on the finding that the restrictions introduced in 2013 had not been sufficiently followed in clinical practice, and cases of serious liver injury still occurred including liver failure.
Evinacumab, sold under the brand name Evkeeza, is a monoclonal antibody medication for the treatment of homozygous familial hypercholesterolemia (HoFH).
Aclidinium bromide/formoterol, sold under the brand names Duaklir and Brimica, is a fixed-dose combination medication for inhalation, used in the management of chronic obstructive pulmonary disease (COPD). It consists of aclidinium bromide, a long-acting muscarinic antagonist, and formoterol, a long-acting β2 agonist.

The Southern African Underwater and Hyperbaric Medical Association (SAUHMA) is an organisation of voluntary members with a special interest in the subject of underwater and/or hyperbaric medicine, recognised by the Council of the South African Medical Association as a special interest group. The Association promotes the practice and facilitates the study of underwater and hyperbaric medicine. Membership includes members and associate members, and may include medical practitioners; registered nurses; registered paramedics; qualified hyperbaric chamber operators; diving instructors; dive operators, and any other person with a special interest underwater or hyperbaric medicine.

A hexavalent vaccine, or 6-in-1 vaccine, is a combination vaccine with six individual vaccines conjugated into one, intended to protect people from multiple diseases. The term usually refers to the children's vaccine that protects against diphtheria, tetanus, pertussis, poliomyelitis, haemophilus B, and hepatitis B, which is used in more than 90 countries around the world including in Europe, Canada, Australia, Jordan, and New Zealand.

The Oxford–AstraZeneca COVID‑19 vaccine, sold under the brand names Covishield and Vaxzevria among others, is a viral vector vaccine for the prevention of COVID-19. It was developed in the United Kingdom by Oxford University and British-Swedish company AstraZeneca, using as a vector the modified chimpanzee adenovirus ChAdOx1. The vaccine is given by intramuscular injection. Studies carried out in 2020 showed that the efficacy of the vaccine is 76.0% at preventing symptomatic COVID-19 beginning at 22 days following the first dose and 81.3% after the second dose. A study in Scotland found that, for symptomatic COVID-19 infection after the second dose, the vaccine is 81% effective against the Alpha variant and 61% against the Delta variant.
Epoetin theta, sold under the brand name Biopoin among others, is a copy of the human hormone erythropoietin.
Sotrovimab, sold under the brand name Xevudy, is a human neutralizing monoclonal antibody with activity against severe acute respiratory syndrome coronavirus 2, known as SARS-CoV-2. It was developed by GlaxoSmithKline and Vir Biotechnology, Inc. Sotrovimab is designed to attach to the spike protein of SARS-CoV-2.