Related Research Articles
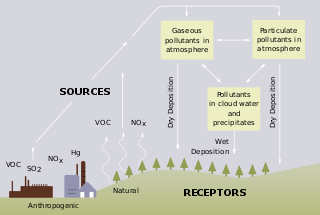
Acid rain is rain or any other form of precipitation that is unusually acidic, meaning that it has elevated levels of hydrogen ions. Most water, including drinking water, has a neutral pH that exists between 6.5 and 8.5, but acid rain has a pH level lower than this and ranges from 4–5 on average. The more acidic the acid rain is, the lower its pH is. Acid rain can have harmful effects on plants, aquatic animals, and infrastructure. Acid rain is caused by emissions of sulphur dioxide and nitrogen oxide, which react with the water molecules in the atmosphere to produce acids.

The nitrogen cycle is the biogeochemical cycle by which nitrogen is converted into multiple chemical forms as it circulates among atmospheric, terrestrial, and marine ecosystems. The conversion of nitrogen can be carried out through both biological and physical processes. Important processes in the nitrogen cycle include fixation, ammonification, nitrification, and denitrification. The majority of Earth's atmosphere (78%) is atmospheric nitrogen, making it the largest source of nitrogen. However, atmospheric nitrogen has limited availability for biological use, leading to a scarcity of usable nitrogen in many types of ecosystems.

Environmental science is an interdisciplinary academic field that integrates physics, biology, and geography to the study of the environment, and the solution of environmental problems. Environmental science emerged from the fields of natural history and medicine during the Enlightenment. Today it provides an integrated, quantitative, and interdisciplinary approach to the study of environmental systems.

Water pollution is the contamination of water bodies, usually as a result of human activities, so that it negatively affects its uses. Water bodies include lakes, rivers, oceans, aquifers, reservoirs and groundwater. Water pollution results when contaminants are introduced into these water bodies. Water pollution can be attributed to one of four sources: sewage discharges, industrial activities, agricultural activities, and urban runoff including stormwater. It can be grouped into surface water pollution or groundwater pollution. For example, releasing inadequately treated wastewater into natural waters can lead to degradation of these aquatic ecosystems. Water pollution can also lead to water-borne diseases for people using polluted water for drinking, bathing, washing or irrigation. Water pollution reduces the ability of the body of water to provide the ecosystem services that it would otherwise provide.

Methylmercury (sometimes methyl mercury) is an organometallic cation with the formula [CH3Hg]+. It is the simplest organomercury compound. Methylmercury is extremely toxic, and its derivatives are the major source of organic mercury for humans. It is a bioaccumulative environmental toxicant.

Human impact on the environment refers to changes to biophysical environments and to ecosystems, biodiversity, and natural resources caused directly or indirectly by humans. Modifying the environment to fit the needs of society is causing severe effects including global warming, environmental degradation, mass extinction and biodiversity loss, ecological crisis, and ecological collapse. Some human activities that cause damage to the environment on a global scale include population growth, overconsumption, overexploitation, pollution, and deforestation. Some of the problems, including global warming and biodiversity loss, have been proposed as representing catastrophic risks to the survival of the human species.
In atmospheric chemistry, NOx is shorthand for nitric oxide and nitrogen dioxide, the nitrogen oxides that are most relevant for air pollution. These gases contribute to the formation of smog and acid rain, as well as affecting tropospheric ozone.

Nonpoint source (NPS) pollution refers to diffuse contamination of water or air that does not originate from a single discrete source. This type of pollution is often the cumulative effect of small amounts of contaminants gathered from a large area. It is in contrast to point source pollution which results from a single source. Nonpoint source pollution generally results from land runoff, precipitation, atmospheric deposition, drainage, seepage, or hydrological modification where tracing pollution back to a single source is difficult. Nonpoint source water pollution affects a water body from sources such as polluted runoff from agricultural areas draining into a river, or wind-borne debris blowing out to sea. Nonpoint source air pollution affects air quality, from sources such as smokestacks or car tailpipes. Although these pollutants have originated from a point source, the long-range transport ability and multiple sources of the pollutant make it a nonpoint source of pollution; if the discharges were to occur to a body of water or into the atmosphere at a single location, the pollution would be single-point.
The Climate Change Science Program (CCSP) was the program responsible for coordinating and integrating research on global warming by U.S. government agencies from February 2002 to June 2009. Toward the end of that period, CCSP issued 21 separate climate assessment reports that addressed climate observations, changes in the atmosphere, expected climate change, impacts and adaptation, and risk management issues. Shortly after President Obama took office, the program's name was changed to U.S. Global Change Research Program (USGCRP) which was also the program's name before 2002. Nevertheless, the Obama Administration generally embraced the CCSP products as sound science providing a basis for climate policy. Because those reports were mostly issued after the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (IPCC), and in some cases focused specifically on the United States, they were generally viewed within the United States as having an importance and scientific credibility comparable to the IPCC assessments for the first few years of the Obama Administration.

Human impact on the nitrogen cycle is diverse. Agricultural and industrial nitrogen (N) inputs to the environment currently exceed inputs from natural N fixation. As a consequence of anthropogenic inputs, the global nitrogen cycle (Fig. 1) has been significantly altered over the past century. Global atmospheric nitrous oxide (N2O) mole fractions have increased from a pre-industrial value of ~270 nmol/mol to ~319 nmol/mol in 2005. Human activities account for over one-third of N2O emissions, most of which are due to the agricultural sector. This article is intended to give a brief review of the history of anthropogenic N inputs, and reported impacts of nitrogen inputs on selected terrestrial and aquatic ecosystems.

Radioecology is the branch of ecology concerning the presence of radioactivity in Earth’s ecosystems. Investigations in radioecology include field sampling, experimental field and laboratory procedures, and the development of environmentally predictive simulation models in an attempt to understand the migration methods of radioactive material throughout the environment.
In the study of air pollution, a critical load is defined as "a quantitative estimate of an exposure to one or more pollutants below which significant harmful effects on specified sensitive elements of the environment do not occur according to present knowledge".

The natural environment, commonly referred to simply as the environment, includes all living and non-living things occurring naturally on Earth.
Landscape limnology is the spatially explicit study of lakes, streams, and wetlands as they interact with freshwater, terrestrial, and human landscapes to determine the effects of pattern on ecosystem processes across temporal and spatial scales. Limnology is the study of inland water bodies inclusive of rivers, lakes, and wetlands; landscape limnology seeks to integrate all of these ecosystem types.

Agricultural pollution refers to biotic and abiotic byproducts of farming practices that result in contamination or degradation of the environment and surrounding ecosystems, and/or cause injury to humans and their economic interests. The pollution may come from a variety of sources, ranging from point source water pollution to more diffuse, landscape-level causes, also known as non-point source pollution and air pollution. Once in the environment these pollutants can have both direct effects in surrounding ecosystems, i.e. killing local wildlife or contaminating drinking water, and downstream effects such as dead zones caused by agricultural runoff is concentrated in large water bodies.

Pollution is an environmental issue in Canada. It has posed health risks to the Canadian population and is an area of concern for Canadian lawmakers. Air, water and soil pollution as well as the associated health effects are prominent points of contention in modern Canadian society.

Freshwater acidification occurs when acidic inputs enter a body of fresh water through the weathering of rocks, invasion of acidifying gas, or by the reduction of acid anions, like sulfate and nitrate within the lake. Freshwater acidification is primarily caused by sulfur oxides (SOx) and nitrogen oxides (NOx) entering the water from atmospheric depositions and soil leaching. Carbonic acid and dissolved carbon dioxide can also enter freshwaters in a similar manner associated with runoff through carbon dioxide-rich soils. Runoff that contains these compounds may be accompanied by acidifying hydrogen ions and inorganic aluminum, which can be toxic to marine organisms. Acid rain is also a contributor to freshwater acidification. It is created when SOx and NOx react with water, oxygen, and other oxidants within the clouds.
The National Atmospheric Deposition Program (NADP) is a Cooperative Research Support Program of the State Agricultural Experiment Stations (NRSP-3). Housed at the Wisconsin State Laboratory of Hygiene at the University of Wisconsin–Madison, the NADP is a collaborative effort between many different groups: Federal, state, tribal, and local governmental agencies, as well as educational institutions, private companies, and non-governmental agencies. These organizations work together in order to operate monitoring sites and report deposition data. The NADP provides free and easy access to all of its data, including seasonal and annual averages, trend plots, deposition maps, reports, manuals, and educational brochures.
References
National Acid Precipitation Assessment Program: Interim Assessment: The Causes and Effects of Acidic Deposition, issued September 17, 1987