
Anisotropy is the structural property of non-uniformity in different directions, as opposed to isotropy. An anisotropic object or pattern has properties that differ according to direction of measurement. For example, many materials exhibit very different physical or mechanical properties when measured along different axes, e.g. absorbance, refractive index, conductivity, and tensile strength.
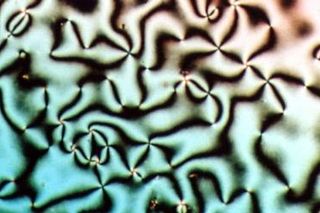
Liquid crystal (LC) is a state of matter whose properties are between those of conventional liquids and those of solid crystals. For example, a liquid crystal can flow like a liquid, but its molecules may be oriented in a common direction as in solid. There are many types of LC phases, which can be distinguished by their optical properties. The contrasting textures arise due to molecules within one area of material ("domain") being oriented in the same direction but different areas having different orientations. An LC material may not always be in an LC state of matter.

In the physical sciences, a phase is a region of material that is chemically uniform, physically distinct, and (often) mechanically separable. In a system consisting of ice and water in a glass jar, the ice cubes are one phase, the water is a second phase, and the humid air is a third phase over the ice and water. The glass of the jar is another separate phase.

In physics, a state of matter is one of the distinct forms in which matter can exist. Four states of matter are observable in everyday life: solid, liquid, gas, and plasma. Many intermediate states are known to exist, such as liquid crystal, and some states only exist under extreme conditions, such as Bose–Einstein condensates and Fermionic condensates, neutron-degenerate matter, and quark–gluon plasma. For a list of exotic states of matter, see the article List of states of matter.

In crystallography, crystal structure is a description of the ordered arrangement of atoms, ions, or molecules in a crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric patterns that repeat along the principal directions of three-dimensional space in matter.

Soft matter or soft condensed matter is a subfield of condensed matter comprising a variety of physical systems that are deformed or structurally altered by thermal or mechanical stress of the magnitude of thermal fluctuations. These materials share an important common feature in that predominant physical behaviors occur at an energy scale comparable with room temperature thermal energy, and that entropy is considered the dominant factor. At these temperatures, quantum aspects are generally unimportant. Soft materials include liquids, colloids, polymers, foams, gels, granular materials, liquid crystals, flesh, and a number of biomaterials. When soft materials interact favorably with surfaces, they become squashed without an external compressive force. Pierre-Gilles de Gennes, who has been called the "founding father of soft matter," received the Nobel Prize in Physics in 1991 for discovering that methods developed for studying order phenomena in simple systems can be generalized to the more complex cases found in soft matter, in particular, to the behaviors of liquid crystals and polymers.
In physics and chemistry, flash freezing is the process whereby objects are rapidly frozen. This is done by subjecting them to cryogenic temperatures, or it can be done through direct contact with liquid nitrogen at −196 °C (−320.8 °F). It is commonly used in the food industry.
The columnar phase is a class of mesophases in which molecules assemble into cylindrical structures to act as mesogens. Originally, these kinds of liquid crystals were called discotic liquid crystals or bowlic liquid crystals because the columnar structures are composed of flat-shaped discotic or bowl-shaped molecules stacked one-dimensionally. Since recent findings provide a number of columnar liquid crystals consisting of non-discoid mesogens, it is more common now to classify this state of matter and compounds with these properties as columnar liquid crystals.
A mesogen is a compound that displays liquid crystal properties. Mesogens can be described as disordered solids or ordered liquids because they arise from a unique state of matter that exhibits both solid- and liquid-like properties called the liquid crystalline state. This liquid crystalline state (LC) is called the mesophase and occurs between the crystalline solid (Cr) state and the isotropic liquid (Iso) state at distinct temperature ranges.

Crystallization is the process by which solids form, where the atoms or molecules are highly organized into a structure known as a crystal. Some ways by which crystals form are precipitating from a solution, freezing, or more rarely deposition directly from a gas. Attributes of the resulting crystal depend largely on factors such as temperature, air pressure, cooling rate, and in the case of liquid crystals, time of fluid evaporation.

Thermochromism is the property of substances to change color due to a change in temperature. A mood ring is an excellent example of this phenomenon, but thermochromism also has more practical uses, such as baby bottles which change to a different color when cool enough to drink, or kettles which change color when water is at or near boiling point. Thermochromism is one of several types of chromism.
In liquid crystals, homeotropic alignment is one of the ways of alignment of liquid crystalline molecules. Homeotropic alignment is the state in which a rod-like liquid crystalline molecule aligns perpendicularly to the substrate. In the polydomain state, the parts also are called homeotropic domains. In contrast, the state in which the molecule aligns to a substance in parallel is called homogeneous alignment.
Liquid crystal polymers (LCPs) are polymers with the property of liquid crystal, usually containing aromatic rings as mesogens. Despite uncrosslinked LCPs, polymeric materials like liquid crystal elastomers (LCEs) and liquid crystal networks (LCNs) can exhibit liquid crystallinity as well. They are both crosslinked LCPs but have different cross link density. They are widely used in the digital display market. In addition, LCPs have unique properties like thermal actuation, anisotropic swelling, and soft elasticity. Therefore, they can be good actuators and sensors. One of the most famous and classical applications for LCPs is Kevlar, a strong but light fiber with wide applications, notably bulletproof vests.

The twisted nematic effect (TN-effect) was a main technology breakthrough that made LCDs practical. Unlike earlier displays, TN-cells did not require a current to flow for operation and used low operating voltages suitable for use with batteries. The introduction of TN-effect displays led to their rapid expansion in the display field, quickly pushing out other common technologies like monolithic LEDs and CRTs for most electronics. By the 1990s, TN-effect LCDs were largely universal in portable electronics, although since then, many applications of LCDs adopted alternatives to the TN-effect such as in-plane switching (IPS) or vertical alignment (VA).

A molecular solid is a solid consisting of discrete molecules. The cohesive forces that bind the molecules together are van der Waals forces, dipole–dipole interactions, quadrupole interactions, π–π interactions, hydrogen bonding, halogen bonding, London dispersion forces, and in some molecular solids, coulombic interactions. Van der Waals, dipole interactions, quadrupole interactions, π–π interactions, hydrogen bonding, and halogen bonding are typically much weaker than the forces holding together other solids: metallic, ionic, and network solids. Intermolecular interactions typically do not involve delocalized electrons, unlike metallic and certain covalent bonds. Exceptions are charge-transfer complexes such as the tetrathiafulvane-tetracyanoquinodimethane (TTF-TCNQ), a radical ion salt. These differences in the strength of force and electronic characteristics from other types of solids give rise to the unique mechanical, electronic, and thermal properties of molecular solids.
A blue phase mode LCD is a liquid crystal display (LCD) technology that uses highly twisted cholesteric phases in a blue phase. It was first proposed in 2007 to obtain a better display of moving images with, for example, frame rates of 100–120 Hz to improve the temporal response of LCDs. This operational mode for LCDs also does not require anisotropic alignment layers and thus theoretically simplifies the LCD manufacturing process.

Solid is one of the four fundamental states of matter along with liquid, gas, and plasma. The molecules in a solid are closely packed together and contain the least amount of kinetic energy. A solid is characterized by structural rigidity and resistance to a force applied to the surface. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire available volume like a gas. The atoms in a solid are bound to each other, either in a regular geometric lattice, or irregularly. Solids cannot be compressed with little pressure whereas gases can be compressed with little pressure because the molecules in a gas are loosely packed.

A liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a nearly constant volume independent of pressure. It is one of the four fundamental states of matter, and is the only state with a definite volume but no fixed shape.
Crystallization of polymers is a process associated with partial alignment of their molecular chains. These chains fold together and form ordered regions called lamellae, which compose larger spheroidal structures named spherulites. Polymers can crystallize upon cooling from melting, mechanical stretching or solvent evaporation. Crystallization affects optical, mechanical, thermal and chemical properties of the polymer. The degree of crystallinity is estimated by different analytical methods and it typically ranges between 10 and 80%, with crystallized polymers often called "semi-crystalline". The properties of semi-crystalline polymers are determined not only by the degree of crystallinity, but also by the size and orientation of the molecular chains.










