In chemistry, a hydride is the anion of hydrogen, H−, or, more commonly, it is a compound in which one or more hydrogen centres have nucleophilic, reducing, or basic properties in it. In compounds that are regarded as hydrides, the hydrogen atom is bonded to a more electropositive element or groups. Compounds containing hydrogen bonded to metals or metalloid may also be referred to as hydrides. Common examples are ammonia (NH3), methane (CH4), ethane (C2H6) (or any other hydrocarbon), and Nickel hydride (NiH), used in NiMH rechargeable batteries.

Oil refinery or petroleum refinery is an industrial process plant where crude oil is transformed and refined into more useful products such as petroleum naphtha, gasoline, diesel fuel, asphalt base, heating oil, kerosene, liquefied petroleum gas, jet fuel and fuel oils. Petrochemicals feed stock like ethylene and propylene can also be produced directly by cracking crude oil without the need of using refined products of crude oil such as naphtha.
Tungsten(VI) fluoride, also known as tungsten hexafluoride, is an inorganic compound with the formula WF6. It is a toxic, corrosive, colorless gas, with a density of about 13 g/L (roughly 11 times heavier than air.) It is one of the densest known gases under standard conditions. WF6 is commonly used by the semiconductor industry to form tungsten films, through the process of chemical vapor deposition. This layer serves as a low-resistivity metallic "interconnect". It is one of seventeen known binary hexafluorides.
A chemical reactor is an enclosed volume in which a chemical reaction takes place. In chemical engineering, it is generally understood to be a process vessel used to carry out a chemical reaction, which is one of the classic unit operations in chemical process analysis. The design of a chemical reactor deals with multiple aspects of chemical engineering. Chemical engineers design reactors to maximize net present value for the given reaction. Designers ensure that the reaction proceeds with the highest efficiency towards the desired output product, producing the highest yield of product while requiring the least amount of money to purchase and operate. Normal operating expenses include energy input, energy removal, raw material costs, labor, etc. Energy changes can come in the form of heating or cooling, pumping to increase pressure, frictional pressure loss or agitation.

A steam explosion is an explosion caused by violent boiling or flashing of water into steam, occurring when water is either superheated, rapidly heated by fine hot debris produced within it, or heated by the interaction of molten metals. Pressure vessels, such as pressurized water (nuclear) reactors, that operate above atmospheric pressure can also provide the conditions for a steam explosion. The water changes from a liquid to a gas with extreme speed, increasing dramatically in volume. A steam explosion sprays steam and boiling-hot water and the hot medium that heated it in all directions, creating a danger of scalding and burning.
The Fischer–Tropsch process is a collection of chemical reactions that converts a mixture of carbon monoxide and hydrogen into liquid hydrocarbons. These reactions occur in the presence of metal catalysts, typically at temperatures of 150–300 °C (302–572 °F) and pressures of one to several tens of atmospheres. The process was first developed by Franz Fischer and Hans Tropsch at the Kaiser-Wilhelm-Institut für Kohlenforschung in Mülheim an der Ruhr, Germany, in 1925.
Dimethylformamide is an organic compound with the formula (CH3)2NC(O)H. Commonly abbreviated as DMF (although this initialism is sometimes used for dimethylfuran, or dimethyl fumarate), this colourless liquid is miscible with water and the majority of organic liquids. DMF is a common solvent for chemical reactions. Dimethylformamide is odorless whereas technical grade or degraded samples often have a fishy smell due to impurity of dimethylamine. Dimethylamine degradation impurities can be removed by sparging degraded samples with an inert gas such as argon or by sonicating the samples under reduced pressure. As its name indicates, it is a derivative of formamide, the amide of formic acid. DMF is a polar (hydrophilic) aprotic solvent with a high boiling point. It facilitates reactions that follow polar mechanisms, such as SN2 reactions.
The water-gas shift reaction (WGSR) describes the reaction of carbon monoxide and water vapor to form carbon dioxide and hydrogen (the mixture of carbon monoxide and hydrogen (not water) is known as water gas):
Catalytic reforming is a chemical process used to convert petroleum refinery naphthas distilled from crude oil into high-octane liquid products called reformates, which are premium blending stocks for high-octane gasoline. The process converts low-octane linear hydrocarbons (paraffins) into branched alkanes (isoparaffins) and cyclic naphthenes, which are then partially dehydrogenated to produce high-octane aromatic hydrocarbons. The dehydrogenation also produces significant amounts of byproduct hydrogen gas, which is fed into other refinery processes such as hydrocracking. A side reaction is hydrogenolysis, which produces light hydrocarbons of lower value, such as methane, ethane, propane and butanes.
Hydrodesulfurization (HDS) is a catalytic chemical process widely used to remove sulfur (S) from natural gas and from refined petroleum products, such as gasoline or petrol, jet fuel, kerosene, diesel fuel, and fuel oils. The purpose of removing the sulfur, and creating products such as ultra-low-sulfur diesel, is to reduce the sulfur dioxide emissions that result from using those fuels in automotive vehicles, aircraft, railroad locomotives, ships, gas or oil burning power plants, residential and industrial furnaces, and other forms of fuel combustion.
In flow chemistry, a chemical reaction is run in a continuously flowing stream rather than in batch production. In other words, pumps move fluid into a tube, and where tubes join one another, the fluids contact one another. If these fluids are reactive, a reaction takes place. Flow chemistry is a well-established technique for use at a large scale when manufacturing large quantities of a given material. However, the term has only been coined recently for its application on a laboratory scale. Often, microreactors are used.
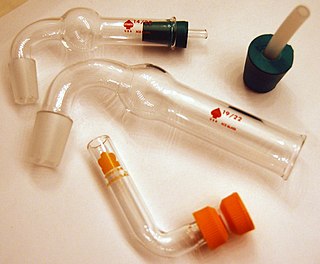
A drying tube or guard tube is a tube-like piece of apparatus used to house a disposable solid desiccant, wherein at one end the tube-like structure terminates in a ground glass joint for use in connecting the drying tube to a reaction vessel, for the purpose of keeping the vessel free of moisture.

Tetralin (1,2,3,4-tetrahydronaphthalene) is a hydrocarbon having the chemical formula C10H12. This molecule is similar to the naphthalene chemical structure except that one ring is saturated.

Cannula transfer or cannulation is a subset of air-free techniques used with a Schlenk line, in transferring liquid or solution samples between reaction vessels via cannulae, avoiding atmospheric contamination. While the syringes are not the same as cannulae, the techniques remain relevant.

FLiBe is a molten salt made from a mixture of lithium fluoride (LiF) and beryllium fluoride (BeF2). It is both a nuclear reactor coolant and solvent for fertile or fissile material. It served both purposes in the Molten-Salt Reactor Experiment (MSRE).
A nuclear reactor coolant is a coolant in a nuclear reactor used to remove heat from the nuclear reactor core and transfer it to electrical generators and the environment. Frequently, a chain of two coolant loops are used because the primary coolant loop takes on short-term radioactivity from the reactor.
Borrowing hydrogen catalysis, also called hydrogen autotransfer, is an important catalytic concept. "Borrowing hydrogen" can be seen as an example of Green chemistry. It is based on the intermediate oxidation of an alcohol substrate to the corresponding aldehyde by the catalyst ; the intermediate aldehyde then reacts with e.g. a secondary amine in a condensation reaction to produce e.g. an imine, that is then reduced by the catalyst in the final step to yield e.g. a tertiary amine. The method is highly atom economic, because is circumvents the activation of the alcohol.
Renewable hydrocarbon fuels via decarboxylation/decarbonylation. With an increasing demand for renewable fuels, extensive research is under way on the utilization of biomass as feedstock for the production of liquid transportation fuels. Using biomass is an attractive alternative, since biomass removes carbon dioxide from the atmosphere as it grows through photosynthesis, thus closing the carbon cycle and making biofuels carbon neutral when certain conditions are met. First generation biofuels such as biodiesel have important drawbacks, as they are normally derived from edible feedstock and are not fully compatible with standard diesel engines. Given that the majority of the problems associated with these fuels stem from their high oxygen content, methods to deoxygenate biomass-derived oils are currently being pursued. The ultimate goal is to convert inedible biomass feeds into hydrocarbon biofuels fully compatible with existing infrastructure. These so-called second generation biofuels can be used as drop-in substitutes for traditional petroleum-derived hydrocarbon fuels.













