
Non-steroidal anti-inflammatory drugs (NSAID) are members of a therapeutic drug class which reduces pain, decreases inflammation, decreases fever, and prevents blood clots. Side effects depend on the specific drug, its dose and duration of use, but largely include an increased risk of gastrointestinal ulcers and bleeds, heart attack, and kidney disease.
An antipyretic is a substance that reduces fever. Antipyretics cause the hypothalamus to override a prostaglandin-induced increase in temperature. The body then works to lower the temperature, which results in a reduction in fever.

Paracetamol (acetaminophen) is a non-opioid analgesic and antipyretic agent used to treat fever and mild to moderate pain. It is a widely used over the counter medication. Common brand names include Tylenol and Panadol.

Ibuprofen is a nonsteroidal anti-inflammatory drug (NSAID) that is used to relieve pain, fever, and inflammation. This includes painful menstrual periods, migraines, and rheumatoid arthritis. It may also be used to close a patent ductus arteriosus in a premature baby. It can be used orally or intravenously. It typically begins working within an hour.
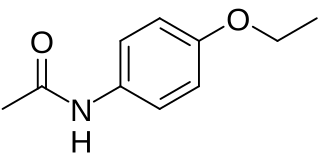
Phenacetin is a pain-relieving and fever-reducing drug, which was widely used following its introduction in 1887. It was withdrawn from medicinal use as dangerous from the 1970s.

Dihydrocodeine is a semi-synthetic opioid analgesic prescribed for pain or severe dyspnea, or as an antitussive, either alone or compounded with paracetamol (acetaminophen) or aspirin. It was developed in Germany in 1908 and first marketed in 1911.

Excedrin is an over-the-counter headache pain reliever, typically in the form of tablets or caplets. It contains paracetamol (acetaminophen), aspirin, and caffeine. It was manufactured by Bristol-Myers Squibb until it was purchased by Novartis in July 2005 along with other products from BMS's over-the-counter business. As of March 2015, GSK holds majority ownership of Excedrin through a joint venture transaction with Novartis. On 18 July 2022, GSK spun off its consumer healthcare business to Haleon.
Butalbital/acetaminophen, sold under the brand name Butapap among others, is a combination medication used to treat tension headaches and migraine headaches. It contains butalbital, a barbiturate and paracetamol (acetaminophen), an analgesic. Versions also containing caffeine are sold under the brand name Fioricet among others. It is taken by mouth. The combination is also sold with codeine.

Phenazone is an analgesic, antipyretic and anti-inflammatory drug. While it predates the term, it is often classified as a nonsteroidal anti-inflammatory drug (NSAID). Phenazone was one of the earliest synthetic medications — when it was patented in 1883, the only synthetic medical chemicals on the market were chloral hydrate, a sedative, trimethylamine, and iodol (tetraiodopyrrol), an early antiseptic. One of the earliest widely used analgesics and antipyretics, phenazone was gradually replaced in common use by other medications including phenacetin, aspirin, paracetamol and modern NSAIDs such as ibuprofen. However, it is still available in several countries either as an over-the-counter or prescribed drug.

Butalbital is a barbiturate with an intermediate duration of action. Butalbital is often combined with other medications, such as paracetamol (acetaminophen) or aspirin, for the treatment of pain and headache. The various formulations combined with codeine are FDA-approved for the treatment of tension headaches. Butalbital has the same chemical formula as talbutal but a different structure—one that presents as 5-allyl-5-isobutylbarbituric acid.

Metamizole or dipyrone is a painkiller, spasm reliever, and fever reliever drug. It is most commonly given by mouth or by intravenous infusion. It belongs to the ampyrone sulfonate family of medicines and was patented in 1922. Metamizole is marketed under various trade names. It was first used medically in Germany under the brand name "Novalgin", and then became widely known in Slavic nations and India under the name "Analgin".

Ethenzamide (2-ethoxybenzamide) is a common analgesic and anti-inflammatory drug that is used for the relief of fever, headaches, and other minor aches and pains. It is also an ingredient in numerous cold medications and many prescription analgesics. It is used as an over-the-counter drug in Japan, often in combination with caffeine and acetaminophen, where it is marketed for uses including toothache, menstrual cramps, headache, and fever.

Analgesic nephropathy is injury to the kidneys caused by analgesic medications such as aspirin, bucetin, phenacetin, and paracetamol. The term usually refers to damage induced by excessive use of combinations of these medications, especially combinations that include phenacetin. It may also be used to describe kidney injury from any single analgesic medication.
Compound analgesics are those with multiple active ingredients; they include many of the stronger prescription analgesics.
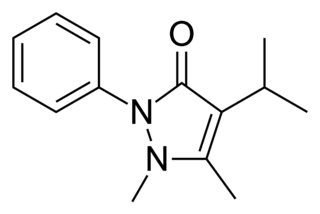
Propyphenazone is a derivative of phenazone with similar analgesic and antipyretic effects. Originally patented in 1931, propyphenazone is marketed as a combination formulation with paracetamol and caffeine for treatment of primary headache disorder.
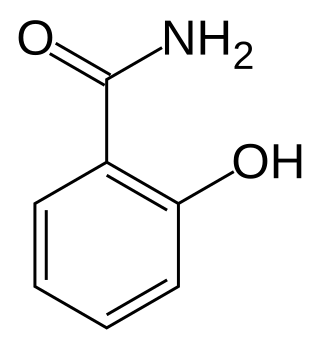
Salicylamide is a non-prescription drug with analgesic and antipyretic properties. Its medicinal uses are similar to those of aspirin. Salicylamide is used in combination with both aspirin and caffeine in the over-the-counter pain remedy PainAid. It was also an ingredient in the over-the-counter pain remedy BC Powder but was removed from the formulation in 2009, and Excedrin used the ingredient from 1960 to 1980 in conjunction with aspirin, acetaminophen, and caffeine. It was used in later formulations of Vincent's powders in Australia as a substitute for phenacetin.
A combination drug or a fixed-dose combination (FDC) is a medicine that includes two or more active ingredients combined in a single dosage form. Terms like "combination drug" or "combination drug product" can be common shorthand for an FDC product, although the latter is more precise if in fact referring to a mass-produced product having a predetermined combination of drugs and respective dosages. And it should also be distinguished from the term "combination product" in medical contexts, which without further specification can refer to products that combine different types of medical products—such as device/drug combinations as opposed to drug/drug combinations. When a combination drug product is a "pill", then it may also be a kind of "polypill" or combopill.
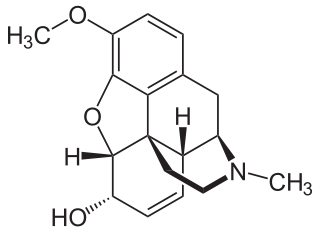
Codeine is an opiate and prodrug of morphine mainly used to treat pain, coughing, and diarrhea. It is also commonly used as a recreational drug. It is found naturally in the sap of the opium poppy, Papaver somniferum. It is typically used to treat mild to moderate degrees of pain. Greater benefit may occur when combined with paracetamol (acetaminophen) or a nonsteroidal anti-inflammatory drug (NSAID) such as aspirin or ibuprofen. Evidence does not support its use for acute cough suppression in children. In Europe, it is not recommended as a cough medicine in those under 12 years of age. It is generally taken by mouth. It typically starts working after half an hour, with maximum effect at two hours. Its effects last for about four to six hours. Codeine exhibits abuse potential similar to other opioid medications, including a risk of habituation and overdose.

Lornoxicam, also known as chlortenoxicam, is a nonsteroidal anti-inflammatory drug (NSAID) of the oxicam class with analgesic, anti-inflammatory and antipyretic properties. It is available in oral and parenteral formulations.













