Related Research Articles

Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray solid which bears a close physical resemblance to the other five elements in the second column of the periodic table: all group 2 elements have the same electron configuration in the outer electron shell and a similar crystal structure.
Electrical resistivity is a fundamental property of a material that measures how strongly it resists electric current. Its inverse, called electrical conductivity, quantifies how well a material conducts electricity. A low resistivity indicates a material that readily allows electric current. Resistivity is commonly represented by the Greek letter ρ (rho). The SI unit of electrical resistivity is the ohm-meter (Ω⋅m). For example, if a 1 m solid cube of material has sheet contacts on two opposite faces, and the resistance between these contacts is 1 Ω, then the resistivity of the material is 1 Ω⋅m.
A phosphor is a substance that exhibits the phenomenon of luminescence; it emits light when exposed to some type of radiant energy. The term is used both for fluorescent or phosphorescent substances which glow on exposure to ultraviolet or visible light, and cathodoluminescent substances which glow when struck by an electron beam in a cathode ray tube.

Sintering or frittage is the process of compacting and forming a solid mass of material by heat or pressure without melting it to the point of liquefaction.

Magnesium oxide (MgO), or magnesia, is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium (see also oxide). It has an empirical formula of MgO and consists of a lattice of Mg2+ ions and O2− ions held together by ionic bonding. Magnesium hydroxide forms in the presence of water (MgO + H2O → Mg(OH)2), but it can be reversed by heating it to remove moisture.

In chemistry, an ionic compound is a chemical compound composed of ions held together by electrostatic forces termed ionic bonding. The compound is neutral overall, but consists of positively charged ions called cations and negatively charged ions called anions. These can be simple ions such as the sodium (Na+) and chloride (Cl−) in sodium chloride, or polyatomic species such as the ammonium (NH+
4) and carbonate (CO2−
3) ions in ammonium carbonate. Individual ions within an ionic compound usually have multiple nearest neighbours, so are not considered to be part of molecules, but instead part of a continuous three-dimensional network. Ionic compounds usually form crystalline structures when solid.

Magnesium carbonate, Mg CO3 (archaic name magnesia alba), is an inorganic salt that is a white solid. Several hydrated and basic forms of magnesium carbonate also exist as minerals.

Magnesium diboride is the inorganic compound with the formula MgB2. It is a dark gray, water-insoluble solid. The compound has attracted attention because it becomes superconducting at 39 K (−234 °C). In terms of its composition, MgB2 differs strikingly from most low-temperature superconductors, which feature mainly transition metals. Its superconducting mechanism is primarily described by BCS theory.

Magnesite is a mineral with the chemical formula MgCO
3. Iron, manganese, cobalt and nickel may occur as admixtures, but only in small amounts.

A refractory material or refractory is a material that is resistant to decomposition by heat, pressure, or chemical attack, and retains strength and form at high temperatures. Refractories are polycrystalline, polyphase, inorganic, non-metallic, porous, and heterogeneous. They are typically composed of oxides or non oxides like carbides, nitrides etc. of the following materials: silicon, aluminium, magnesium, calcium, and zirconium. Some metals with melting points >1850 °C like niobium, chromium, zirconium, tungsten, rhenium, tantalum etc. are also considered as refractories.

Dolomite (also known as dolomite rock, dolostone or dolomitic rock) is a sedimentary carbonate rock that contains a high percentage of the mineral dolomite, CaMg(CO3)2. In old USGS publications, it was referred to as magnesian limestone, a term now reserved for magnesium-deficient dolomites or magnesium-rich limestones. Dolomite has a stoichiometric ratio of nearly equal amounts of magnesium and calcium. Most dolomite rock formed as a magnesium replacement of limestone or lime mud before lithification. Dolomite rock is resistant to erosion and can either contain bedded layers or be unbedded. It is less soluble than limestone in weakly acidic groundwater, but it can still develop solution features (karst) over time. Dolomite rock can act as an oil and natural gas reservoir.
Precipitation hardening, also called age hardening or particle hardening, is a heat treatment technique used to increase the yield strength of malleable materials, including most structural alloys of aluminium, magnesium, nickel, titanium, and some steels and stainless steels. In superalloys, it is known to cause yield strength anomaly providing excellent high-temperature strength.
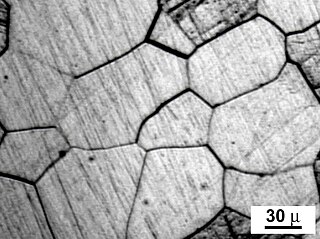
A grain boundary is the interface between two grains, or crystallites, in a polycrystalline material. Grain boundaries are 2D defects in the crystal structure, and tend to decrease the electrical and thermal conductivity of the material. Most grain boundaries are preferred sites for the onset of corrosion and for the precipitation of new phases from the solid. They are also important to many of the mechanisms of creep. On the other hand, grain boundaries disrupt the motion of dislocations through a material, so reducing crystallite size is a common way to improve mechanical strength, as described by the Hall–Petch relationship. The study of grain boundaries and their effects on the mechanical, electrical and other properties of materials forms an important topic in materials science.

The Pidgeon process is one of the methods of magnesium metal production, via a silicothermic reduction. Practical production requires roughly 35–40 MWh/ton of metal produced, which is on par with the molten salt electrolytic methods of production, though above the 7 MWh/ton theoretical minimum.
Kröger–Vink notation is a set of conventions that are used to describe electric charges and lattice positions of point defect species in crystals. It is primarily used for ionic crystals and is particularly useful for describing various defect reactions. It was proposed by F. A. Kröger and H. J. Vink.

Vanadium nitride, VN, is a chemical compound of vanadium and nitrogen.
Eco-Cement is a brand-name for a type of cement which incorporates reactive magnesia, another hydraulic cement such as Portland cement, and optionally pozzolans and industrial by-products, to reduce the environmental impact relative to conventional cement. One problem with the commercialization of this cement, other than the conservatism of the building industry, is that the feedstock magnesite is rarely mined.

Zirconium diboride (ZrB2) is a highly covalent refractory ceramic material with a hexagonal crystal structure. ZrB2 is an ultra high temperature ceramic (UHTC) with a melting point of 3246 °C. This along with its relatively low density of ~6.09 g/cm3 (measured density may be higher due to hafnium impurities) and good high temperature strength makes it a candidate for high temperature aerospace applications such as hypersonic flight or rocket propulsion systems. It is an unusual ceramic, having relatively high thermal and electrical conductivities, properties it shares with isostructural titanium diboride and hafnium diboride.
Chlorine gas can be produced by extracting from natural materials, including the electrolysis of a sodium chloride solution (brine) and other ways.
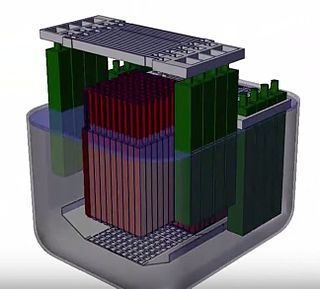
The stable salt reactor (SSR) is a nuclear reactor design under development by Moltex Energy Ltd, based in the United Kingdom and Canada.