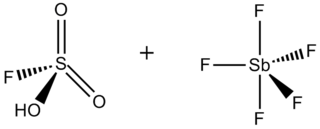In organic chemistry, an alkane, or paraffin (a historical name that also has other meanings), is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in which all the carbon–carbon bonds are single. Alkanes have the general chemical formula CnH2n+2. The alkanes range in complexity from the simplest case of methane (CH4), where n = 1 (sometimes called the parent molecule), to arbitrarily large and complex molecules, like pentacontane (C50H102) or 6-ethyl-2-methyl-5-(1-methylethyl) octane, an isomer of tetradecane (C14H30).

In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula CnH2n−2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic but tend to be more reactive.

A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei, and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive elements where both electronic and nuclear changes can occur.

In chemistry, a ketone is an organic compound with the structure R(C=O)R', where R and R' can be a variety of carbon-containing substituents. Ketones and aldehydes are simple compounds that contain a carbonyl group. They are considered "simple" because they do not have reactive groups like −OH or −Cl attached directly to the carbon atom in the carbonyl group, as in carboxylic acids containing −COOH. Many ketones are known and many are of great importance in industry and in biology. Examples include many sugars (ketoses) and the industrial solvent acetone, which is the smallest ketone.

The haloalkanes are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and commercial names. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes which contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula "RX" where R is an alkyl or substituted alkyl group and X is a halogen.

In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a carbonyl group is often referred to as a carbonyl compound.
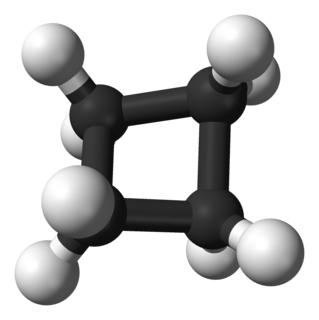
In organic chemistry, the cycloalkanes are the monocyclic saturated hydrocarbons. In other words, a cycloalkane consists only of hydrogen and carbon atoms arranged in a structure containing a single ring, and all of the carbon-carbon bonds are single. Cycloalkanes are named analogously to their normal alkane counterparts of the same carbon count: cyclopropane, cyclobutane, cyclopentane, cyclohexane, etc. The larger cycloalkanes, with more than 20 carbon atoms are typically called cycloparaffins.

In organic chemistry, an alkyl substituent is an alkane missing one hydrogen. The term alkyl is intentionally unspecific to include many possible substitutions. An acyclic alkyl has the general formula CnH2n+1. A cycloalkyl is derived from a cycloalkane by removal of a hydrogen atom from a ring and has the general formula CnH2n-1. Typically an alkyl is a part of a larger molecule. In structural formula, the symbol R is used to designate a generic (unspecified) alkyl group. The smallest alkyl group is methyl, with the formula CH3−. The name 'alkyl' is strictly given to straight chain groups only; for branched chain groups, terms like "iso" or "neo" are often used along with the word "alkyl".
Cyclopropane is a cycloalkane molecule with the molecular formula C3H6, consisting of three carbon atoms linked to each other to form a ring, with each carbon atom bearing two hydrogen atoms resulting in D3h molecular symmetry. The small size of the ring creates substantial ring strain in the structure.

An alicyclic compound is an organic compound that is both aliphatic and cyclic. They contain one or more all-carbon rings which may be either saturated or unsaturated, but do not have aromatic character. Alicyclic compounds may have one or more aliphatic side chains attached.
Cyclopentane is a highly flammable alicyclic hydrocarbon with chemical formula C5H10 and CAS number 287-92-3, consisting of a ring of five carbon atoms each bonded with two hydrogen atoms above and below the plane. It occurs as a colorless liquid with a petrol-like odor. Its melting point is −94 °C and its boiling point is 49 °C. Cyclopentane is in the class of cycloalkanes, being alkanes that have one or more rings of carbon atoms. It is formed by cracking cyclohexane in the presence of alumina at a high temperature and pressure.

A Newman projection, useful in alkane stereochemistry, visualizes the conformation of a chemical bond from front to back, with the front atom represented by a dot and the back carbon as a circle. The front carbon atom is called proximal, while the back atom is called distal. This type of representation clearly illustrates the specific dihedral angle between the proximal and distal atoms.
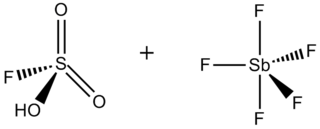
Magic acid (FSO3H·SbF5) is a superacid consisting of a mixture, most commonly in a 1:1 molar ratio, of fluorosulfuric acid (HSO3F) and antimony pentafluoride (SbF5). This conjugate Brønsted–Lewis superacid system was developed in the 1960s by the George Olah lab at Case Western Reserve University, and has been used to stabilize carbocations and hypercoordinated carbonium ions in liquid media. Magic acid and other superacids are also used to catalyze isomerization of saturated hydrocarbons, and have been shown to protonate even weak bases, including methane, xenon, halogens, and molecular hydrogen.
Higher alkanes are alkanes having nine or more carbon atoms. Nonane is the lightest alkane to have a flash point above 25 °C, and is not classified as dangerously flammable.

A quaternary carbon is a carbon atom bound to four other carbon atoms. For this reason, quaternary carbon atoms are found only in hydrocarbons having at least five carbon atoms. Quaternary carbon atoms can occur in branched alkanes, but not in linear alkanes.

A tertiary carbon atom is a carbon atom bound to three other carbon atoms. For this reason, tertiary carbon atoms are found only in hydrocarbons containing at least four carbon atoms. Tertiary carbon atoms can occur, for example, in branched alkanes, but not in linear alkanes.
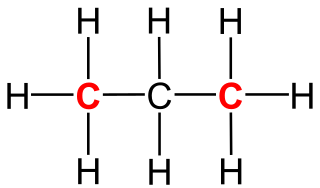
A primary carbon is a carbon atom which is bound to only one other carbon atom. It is thus at the end of a carbon chain. In case of an alkane, three hydrogen atoms are bound to a primary carbon. A hydrogen atom could also be replaced by a hydroxy group, which would make the molecule a primary alcohol.

2-Methylpentane, trivially known as isohexane, is a branched-chain alkane with the molecular formula C6H14. It is a structural isomer of hexane composed of a methyl group bonded to the second carbon atom in a pentane chain.
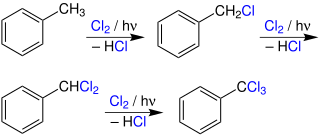
Photochlorination is a chemical reaction which is initiated by light, in which either hydrogen is replaced by chlorine in a hydrocarbon compound or chlorine is reacted via an addition reaction to an aromatic or olefinic hydrocarbon. Photochlorination is carried out in addition to thermal and catalytic chlorination on an industrial scale, usually in the liquid phase, sometimes in the presence of inert solvents. The process is exothermic and proceeds as a chain reaction initiated by the homolytic cleavage of molecular chlorine into chlorine radicals by ultraviolet radiation.