Selenium sulfide can refer to either of the following:
- Selenium disulfide, SeS2
- Selenium hexasulfide, Se2S6
Selenium sulfide can refer to either of the following:

The chalcogens are the chemical elements in group 16 of the periodic table. This group is also known as the oxygen family. Group 16 consists of the elements oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and the radioactive elements polonium (Po) and livermorium (Lv). Often, oxygen is treated separately from the other chalcogens, sometimes even excluded from the scope of the term "chalcogen" altogether, due to its very different chemical behavior from sulfur, selenium, tellurium, and polonium. The word "chalcogen" is derived from a combination of the Greek word khalkόs (χαλκός) principally meaning copper, and the Latinized Greek word genēs, meaning born or produced.

Selenium is a chemical element; it has the symbol Se and atomic number 34. It is a nonmetal with properties that are intermediate between the elements above and below in the periodic table, sulfur and tellurium, and also has similarities to arsenic. It seldom occurs in its elemental state or as pure ore compounds in Earth's crust. Selenium was discovered in 1817 by Jöns Jacob Berzelius, who noted the similarity of the new element to the previously discovered tellurium.
SLA may refer to:

Lake Koocanusa is a reservoir in British Columbia (Canada) and Montana formed by the damming of the Kootenai River by the Libby Dam in 1972. The Dam was formally dedicated by President Gerald Ford on August 24, 1975.

Hydrogen selenide is an inorganic compound with the formula H2Se. This hydrogen chalcogenide is the simplest and most commonly encountered hydride of selenium. H2Se is a colorless, flammable gas under standard conditions. It is the most toxic selenium compound with an exposure limit of 0.05 ppm over an 8-hour period. Even at extremely low concentrations, this compound has a very irritating smell resembling that of decayed horseradish or 'leaking gas', but smells of rotten eggs at higher concentrations.
Selenium disulfide, also known as selenium sulfide, is a chemical compound and medication used to treat seborrheic dermatitis, dandruff, and pityriasis versicolor. It is applied to the affected area as a lotion or shampoo. Symptoms frequently return if treatment is stopped.

Selenium dioxide is the chemical compound with the formula SeO2. This colorless solid is one of the most frequently encountered compounds of selenium.

Sodium selenite is the inorganic compound with the formula Na2SeO3. This salt is a colourless solid. The pentahydrate Na2SeO3(H2O)5 is the most common water-soluble selenium compound.
Organoselenium chemistry is the science exploring the properties and reactivity of organoselenium compounds, chemical compounds containing carbon-to-selenium chemical bonds. Selenium belongs with oxygen and sulfur to the group 16 elements or chalcogens, and similarities in chemistry are to be expected. Organoselenium compounds are found at trace levels in ambient waters, soils and sediments.
Selenium is an open source umbrella project for a range of tools and libraries aimed at supporting browser automation. It provides a playback tool for authoring functional tests across most modern web browsers, without the need to learn a test scripting language. It also provides a test domain-specific language (Selenese) to write tests in a number of popular programming languages, including JavaScript (Node.js), C#, Groovy, Java, Perl, PHP, Python, Ruby and Scala. Selenium runs on Windows, Linux, and macOS. It is open-source software released under the Apache License 2.0.
Keshan disease is a congestive cardiomyopathy caused by a combination of dietary deficiency of selenium and the presence of a mutated strain of Coxsackievirus, named after Keshan County of Heilongjiang province, Northeast China, where symptoms were first noted. These symptoms were later found prevalent in a wide belt extending from northeast to southwest China, all due to selenium-deficient soil. The disease peaked in 1960–1970, killing thousands of people.
Selenium oxide may refer to either of the following compounds:
Selenite may refer to:
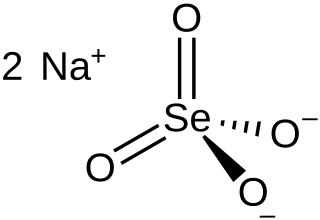
Sodium selenate is the inorganic compound with the formula Na
2SeO
4, not to be confused with sodium selenite. It exists as the anhydrous salt, the heptahydrate, and the decahydrate. These are white, water-soluble solids. The decahydrate is a common ingredient in multivitamins and livestock feed as a source of selenium. The anhydrous salt is used in the production of some glass. Although the selenates are much more toxic, many physical properties of sodium selenate and sodium sulfate are similar.
Selenium chloride may refer to either of the following:
Selenium fluoride may refer to:
Diselenide may refer to:

Selenium is an essential micronutrient for animals, though it is toxic in large doses. In plants, it sometimes occurs in toxic amounts as forage, e.g. locoweed. Selenium is a component of the amino acids selenocysteine and selenomethionine. In humans, selenium is a trace element nutrient that functions as cofactor for glutathione peroxidases and certain forms of thioredoxin reductase. Selenium-containing proteins are produced from inorganic selenium via the intermediacy of selenophosphate (PSeO33−).
34 may refer to:
Selen may refer to