
Adenylyl cyclase is an enzyme with key regulatory roles in essentially all cells. It is the most polyphyletic known enzyme: six distinct classes have been described, all catalyzing the same reaction but representing unrelated gene families with no known sequence or structural homology. The best known class of adenylyl cyclases is class III or AC-III. AC-III occurs widely in eukaryotes and has important roles in many human tissues.

G protein-coupled receptors (GPCRs), also known as seven-(pass)-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptors, and G protein-linked receptors (GPLR), form a large group of evolutionarily-related proteins that are cell surface receptors that detect molecules outside the cell and activate cellular responses. Coupling with G proteins, they are called seven-transmembrane receptors because they pass through the cell membrane seven times. Ligands can bind either to extracellular N-terminus and loops or to the binding site within transmembrane helices. They are all activated by agonists although a spontaneous auto-activation of an empty receptor can also be observed.

A protein kinase is a kinase which selectively modifies other proteins by covalently adding phosphates to them (phosphorylation) as opposed to kinases which modify lipids, carbohydrates, or other molecules. Phosphorylation usually results in a functional change of the target protein (substrate) by changing enzyme activity, cellular location, or association with other proteins. The human genome contains about 500 protein kinase genes and they constitute about 2% of all human genes. There are two main types of protein kinase, the great majority are serine/threonine kinases, which phosphorylate the hydroxyl groups of serines and threonines in their targets and the other are tyrosine kinases, although additional types exist. Protein kinases are also found in bacteria and plants. Up to 30% of all human proteins may be modified by kinase activity, and kinases are known to regulate the majority of cellular pathways, especially those involved in signal transduction.

Rhodopsin is a light-sensitive receptor protein involved in visual phototransduction. It is named after ancient Greek ῥόδον for rose, due to its pinkish color, and ὄψις for sight. Rhodopsin is a biological pigment found in the rods of the retina and is a G-protein-coupled receptor (GPCR). It belongs to opsins. Rhodopsin is extremely sensitive to light, and thus enables vision in low-light conditions. When rhodopsin is exposed to light, it immediately photobleaches. In humans, it is regenerated fully in about 30 minutes, after which rods are more sensitive.

Proteorhodopsin is a family of over 50 photoactive retinylidene proteins, a larger family of transmembrane proteins that use retinal as a chromophore for light-mediated functionality, in this case, a proton pump. Some homologues exist as pentamers or hexamers. pRhodopsin is found in marine planktonic bacteria, archaea and eukaryotes (protae), but was first discovered in bacteria.

Opsins are a group of proteins made light-sensitive via the chromophore retinal found in photoreceptor cells of the retina. Five classical groups of opsins are involved in vision, mediating the conversion of a photon of light into an electrochemical signal, the first step in the visual transduction cascade. Another opsin found in the mammalian retina, melanopsin, is involved in circadian rhythms and pupillary reflex but not in vision.
Halobacterium is a genus in the family Halobacteriaceae.

G protein-coupled receptor kinases are a family of protein kinases within the AGC group of kinases. Like all AGC kinases, GRKs use ATP to add phosphate to Serine and Threonine residues in specific locations of target proteins. In particular, GRKs phosphorylate intracellular domains of G protein-coupled receptors (GPCRs). GRKs function in tandem with arrestin proteins to regulate the sensitivity of GPCRs for stimulating downstream heterotrimeric G protein and G protein-independent signaling pathways.

Recoverin is a 23 kilodalton (kDa) neuronal calcium-binding protein that is primarily detected in the photoreceptor cells of the eye. It plays a key role in the inhibition of rhodopsin kinase, a molecule which regulates the phosphorylation of rhodopsin. A reduction in this inhibition helps regulate sensory adaptation in the retina, since the light-dependent channel closure in photoreceptors causes calcium levels to decrease, which relieves the inhibition of rhodopsin kinase by calcium-bound recoverin, leading to a more rapid inactivation of metarhodopsin II.
Halorhodopsin is a light-gated ion pump, specific for chloride ions, found in archaea, known as halobacteria. It is a seven-transmembrane retinylidene protein from microbial rhodopsin family. It is similar in tertiary structure to vertebrate rhodopsins, the pigments that sense light in the retina. Halorhodopsin also shares sequence similarity to channelrhodopsin, another light-driven ion channel. Halorhodopsin contains the essential light-isomerizable vitamin A derivative all-trans-retinal. Due to the intense attention on solving the structure and function of this molecule, halorhodopsin is one of the few membrane proteins whose crystal structure is known.
The formyl peptide receptors (FPR) belong to a class of G protein-coupled receptors involved in chemotaxis. In humans, there are three formyl peptide receptor isoforms, each encoded by a separate gene that are named FPR1, FPR2, and FPR3. These receptors were originally identified by their ability to bind N-formyl peptides such as N-formylmethionine produced by the degradation of either bacterial or host cells. Hence formyl peptide receptors are involved in mediating immune cell response to infection. These receptors may also act to suppress the immune system under certain conditions. The close phylogenetic relation of signaling in chemotaxis and olfaction was recently proved by detection formyl peptide receptor like proteins as a distinct family of vomeronasal organ chemosensors in mice.
Retinylidene protein, is a family of proteins that use retinal as a chromophore for light reception. It is the molecular basis for a variety of light-sensing systems from phototaxis in flagellates to eyesight in animals. Retinylidene proteins include all forms of opsin and rhodopsin. While rhodopsin in the narrow sense refers to a dim-light visual pigment found in vertebrates, usually on rod cells, rhodopsin in the broad sense refers any molecule consisting of an opsin and a retinal chromophore in the ground state. When activated by light, the chromophore is isomerized, at which point the molecule as a whole is no longer rhodopsin, but a related molecule such as metarhodopsin. However, it remains a retinylidene protein. The chromophore then separates from the opsin, at which point the bare opsin is a retinylidene protein. Thus, the molecule remains a retinylidene protein throughout the phototransduction cycle.

Histidine kinases (HK) are multifunctional, and in non-animal kingdoms, typically transmembrane, proteins of the transferase class of enzymes that play a role in signal transduction across the cellular membrane. The vast majority of HKs are homodimers that exhibit autokinase, phosphotransfer, and phosphatase activity. HKs can act as cellular receptors for signaling molecules in a way analogous to tyrosine kinase receptors (RTK). Multifunctional receptor molecules such as HKs and RTKs typically have portions on the outside of the cell that bind to hormone- or growth factor-like molecules, portions that span the cell membrane, and portions within the cell that contain the enzymatic activity. In addition to kinase activity, the intracellular domains typically have regions that bind to a secondary effector molecule or complex of molecules that further propagate signal transduction within the cell. Distinct from other classes of protein kinases, HKs are usually parts of a two-component signal transduction mechanisms in which HK transfers a phosphate group from ATP to a histidine residue within the kinase, and then to an aspartate residue on the receiver domain of a response regulator protein. More recently, the widespread existence of protein histidine phosphorylation distinct from that of two-component histidine kinases has been recognised in human cells. In marked contrast to Ser, Thr and Tyr phosphorylation, the analysis of phosphorylated Histidine using standard biochemical and mass spectrometric approaches is much more challenging, and special procedures and separation techniques are required for their preservation alongside classical Ser, Thr and Tyr phosphorylation on proteins isolated from human cells.

Dishevelled (Dsh) is a family of proteins involved in canonical and non-canonical Wnt signalling pathways. Dsh is a cytoplasmic phosphoprotein that acts directly downstream of frizzled receptors. It takes its name from its initial discovery in flies, where a mutation in the dishevelled gene was observed to cause improper orientation of body and wing hairs. There are vertebrate homologs in zebrafish, Xenopus (Xdsh), mice and humans. Dsh relays complex Wnt signals in tissues and cells, in normal and abnormal contexts. It is thought to interact with the novel protein, SPATS1, when regulating the Wnt Signalling pathway.

Cell surface receptors are receptors that are embedded in the plasma membrane of cells. They act in cell signaling by receiving extracellular molecules. They are specialized integral membrane proteins that allow communication between the cell and the extracellular space. The extracellular molecules may be hormones, neurotransmitters, cytokines, growth factors, cell adhesion molecules, or nutrients; they react with the receptor to induce changes in the metabolism and activity of a cell. In the process of signal transduction, ligand binding affects a cascading chemical change through the cell membrane.
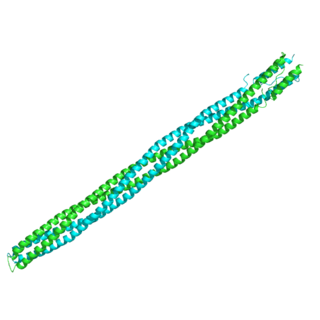
The Methyl-accepting chemotaxis proteins are a family of transmembrane receptors that mediate chemotactic response in certain enteric bacteria, such as Salmonella typhimurium and Escherichia coli. These methyl-accepting chemotaxis receptors are one of the first components in the sensory excitation and adaptation responses in bacteria, which act to alter swimming behaviour upon detection of specific chemicals. Use of the MCP allows bacteria to detect concentrations of molecules in the extracellular matrix so that the bacteria may smooth swim or tumble accordingly. If the bacterium detects rising levels of attractants (nutrients) or declining levels of repellents (toxins), the bacterium will continue swimming forward, or smooth swimming. If the bacterium detects declining levels of attractants or rising levels of repellents, the bacterium will tumble and re-orient itself in a new direction. In this manner, a bacterium may swim towards nutrients and away from toxins
In molecular biology, the HAMP domain is an approximately 50-amino acid alpha-helical region that forms a dimeric, four-helical coiled coil. It is found in bacterial sensor and chemotaxis proteins and in eukaryotic histidine kinases. The bacterial proteins are usually integral membrane proteins and part of a two-component signal transduction pathway. One or several copies of the HAMP domain can be found in association with other domains, such as the histidine kinase domain, the bacterial chemotaxis sensory transducer domain, the PAS repeat, the EAL domain, the GGDEF domain, the protein phosphatase 2C-like domain, the guanylate cyclase domain, or the response regulatory domain. In its most common setting, the HAMP domain transmits conformational changes in periplasmic ligand-binding domains to cytoplasmic signalling kinase and methyl-acceptor domains and thus regulates the phosphorylation or methylation activity of homodimeric receptors.

Tyrosine phosphorylation is the addition of a phosphate (PO43−) group to the amino acid tyrosine on a protein. It is one of the main types of protein phosphorylation. This transfer is made possible through enzymes called tyrosine kinases. Tyrosine phosphorylation is a key step in signal transduction and the regulation of enzymatic activity.

Microbial rhodopsins, also known as bacterial rhodopsins are retinal-binding proteins that provide light-dependent ion transport and sensory functions in halophilic and other bacteria. They are integral membrane proteins with seven transmembrane helices, the last of which contains the attachment point for retinal.
Archaerhodopsin proteins are a family of retinal-containing photoreceptors found in the archaea genera Halobacterium and Halorubrum. Like the homologous bacteriorhodopsin (bR) protein, archaerhodopsins harvest energy from sunlight to pump H+ ions out of the cell, establishing a proton motive force that is used for ATP synthesis. They have some structural similarities to the mammalian GPCR protein rhodopsin, but are not true homologs.














