
In genetics and developmental biology, somatic cell nuclear transfer (SCNT) is a laboratory strategy for creating a viable embryo from a body cell and an egg cell. The technique consists of taking an enucleated oocyte and implanting a donor nucleus from a somatic (body) cell. It is used in both therapeutic and reproductive cloning. In 1996, Dolly the sheep became famous for being the first successful case of the reproductive cloning of a mammal. In January 2018, a team of scientists in Shanghai announced the successful cloning of two female crab-eating macaques from foetal nuclei.
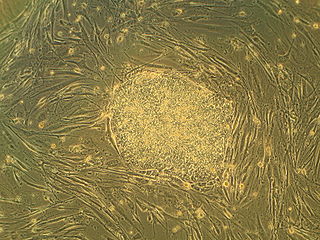
Embryonic stem cells (ESCs) are pluripotent stem cells derived from the inner cell mass of a blastocyst, an early-stage pre-implantation embryo. Human embryos reach the blastocyst stage 4–5 days post fertilization, at which time they consist of 50–150 cells. Isolating the inner cell mass (embryoblast) using immunosurgery results in destruction of the blastocyst, a process which raises ethical issues, including whether or not embryos at the pre-implantation stage have the same moral considerations as embryos in the post-implantation stage of development.

The Wisconsin Alumni Research Foundation is the independent nonprofit technology transfer organization serving the University of Wisconsin–Madison and Morgridge Institute for Research. It provides significant research support, granting tens of millions of dollars to the university each year and contributing to the university's "margin of excellence".

James Alexander Thomson is an American developmental biologist best known for deriving the first human embryonic stem cell line in 1998 and for deriving human induced pluripotent stem cells (iPS) in 2007.

A stem cell line is a group of stem cells that is cultured in vitro and can be propagated indefinitely. Stem cell lines are derived from either animal or human tissues and come from one of three sources: embryonic stem cells, adult stem cells, or induced stem cells. They are commonly used in research and regenerative medicine.
The stem cell controversy is the consideration of the ethics of research involving the development and use of human embryos. Most commonly, this controversy focuses on embryonic stem cells. Not all stem cell research involves human embryos. For example, adult stem cells, amniotic stem cells, and induced pluripotent stem cells do not involve creating, using, or destroying human embryos, and thus are minimally, if at all, controversial. Many less controversial sources of acquiring stem cells include using cells from the umbilical cord, breast milk, and bone marrow, which are not pluripotent.

Molecular cytogenetics combines two disciplines, molecular biology and cytogenetics, and involves the analysis of chromosome structure to help distinguish normal and cancer-causing cells. Human cytogenetics began in 1956 when it was discovered that normal human cells contain 46 chromosomes. However, the first microscopic observations of chromosomes were reported by Arnold, Flemming, and Hansemann in the late 1800s. Their work was ignored for decades until the actual chromosome number in humans was discovered as 46. In 1879, Arnold examined sarcoma and carcinoma cells having very large nuclei. Today, the study of molecular cytogenetics can be useful in diagnosing and treating various malignancies such as hematological malignancies, brain tumors, and other precursors of cancer. The field is overall focused on studying the evolution of chromosomes, more specifically the number, structure, function, and origin of chromosome abnormalities. It includes a series of techniques referred to as fluorescence in situ hybridization, or FISH, in which DNA probes are labeled with different colored fluorescent tags to visualize one or more specific regions of the genome. Introduced in the 1980s, FISH uses probes with complementary base sequences to locate the presence or absence of the specific DNA regions you are looking for. FISH can either be performed as a direct approach to metaphase chromosomes or interphase nuclei. Alternatively, an indirect approach can be taken in which the entire genome can be assessed for copy number changes using virtual karyotyping. Virtual karyotypes are generated from arrays made of thousands to millions of probes, and computational tools are used to recreate the genome in silico.

The University of Wisconsin School of Medicine and Public Health (UWSMPH) is a professional school for the study of medicine and public health at the University of Wisconsin–Madison. It is one of only two medical schools in Wisconsin, along with the Medical College of Wisconsin in Milwaukee, and the only public one.

Induced pluripotent stem cells are a type of pluripotent stem cell that can be generated directly from a somatic cell. The iPSC technology was pioneered by Shinya Yamanaka's lab in Kyoto, Japan, who showed in 2006 that the introduction of four specific genes, collectively known as Yamanaka factors, encoding transcription factors could convert somatic cells into pluripotent stem cells. He was awarded the 2012 Nobel Prize along with Sir John Gurdon "for the discovery that mature cells can be reprogrammed to become pluripotent."
A biorepository is a facility that collects, catalogs, and stores samples of biological material for laboratory research. Biorepositories collect and manage specimens from animals, plants, and other living organisms. Biorepositories store many different types of specimens, including samples of blood, urine, tissue, cells, DNA, RNA, and proteins. If the samples are from people, they may be stored with medical information along with written consent to use the samples in laboratory studies.
Virtual karyotype is the digital information reflecting a karyotype, resulting from the analysis of short sequences of DNA from specific loci all over the genome, which are isolated and enumerated. It detects genomic copy number variations at a higher resolution for level than conventional karyotyping or chromosome-based comparative genomic hybridization (CGH). The main methods used for creating virtual karyotypes are array-comparative genomic hybridization and SNP arrays.
Junying Yu is a Chinese stem cell biologist. She is a researcher at the University of Wisconsin–Madison.
Stem cell laws are the law rules, and policy governance concerning the sources, research, and uses in treatment of stem cells in humans. These laws have been the source of much controversy and vary significantly by country. In the European Union, stem cell research using the human embryo is permitted in Sweden, Spain, Finland, Belgium, Greece, Britain, Denmark and the Netherlands; however, it is illegal in Germany, Austria, Ireland, Italy, and Portugal. The issue has similarly divided the United States, with several states enforcing a complete ban and others giving support. Elsewhere, Japan, India, Iran, Israel, South Korea, China, and Australia are supportive. However, New Zealand, most of Africa, and most of South America are restrictive.
Stem cell laws and policy in the United States have had a complicated legal and political history.
Su-Chun Zhang, is an American stem cell researcher at Duke-NUS Medical School in Singapore and the University of Wisconsin–Madison.

The University of Wisconsin Carbone Cancer Center (UWCCC) is a comprehensive cancer center in Wisconsin, as designated by the National Cancer Institute (NCI), the lead federal agency for cancer research. It is an integral part of both the University of Wisconsin (UW) and the University of Wisconsin Hospital and Clinics. It is located in Madison, Wisconsin.
The Morgridge Institute for Research is a private, nonprofit biomedical research institute in Madison, Wis., affiliated with the University of Wisconsin–Madison. The institute works to improve human health by conducting, enabling and translating interdisciplinary biomedical research. Research in regenerative biology, virology, medical devices and core computational technology is currently underway.

George Quentin Daley is the Dean of the Faculty of Medicine, Caroline Shields Walker Professor of Medicine, and Professor of Biological Chemistry and Molecular Pharmacology at Harvard Medical School. He was formerly the Robert A. Stranahan Professor of Pediatrics at Harvard Medical School, Director of the Stem Cell Transplantation Program at Boston Children's Hospital, and an investigator of the Howard Hughes Medical Institute, Associate Director of Children’s Stem Cell Program, a member of the Executive Committee of the Harvard Stem Cell Institute. He is a past president of the International Society for Stem Cell Research (2007–2008).
The Coriell Institute for Medical Research is an independent, non-profit biomedical research center dedicated to the study of the human genome. Coriell features programs in biobanking, personalized medicine, cell biology, cytogenetics, genotyping, and induced pluripotent stem cell science. Located in downtown Camden, New Jersey, the Institute has partnered with several prominent state and national health leaders, including Cooper University Hospital, the Cooper Medical School of Rowan University, the United States Air Force, the University of Pennsylvania, and Stanford University.
Biological Industries is an Israel multinational, chemicals and biotechnology company, headquartered in Israel, with R&D and scientific training centers in Europe and the United States. The company provides product development and cell culture services to the pharmaceutical and biological industries, including custom manufacturing of biopharmaceuticals, and custom cell culture media formulations.










