In organic chemistry, amines (, UK also ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; see Category:Amines for a list of amines. Inorganic derivatives of ammonia are also called amines, such as monochloramine (NClH2).

In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis.
Hydrolysis is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
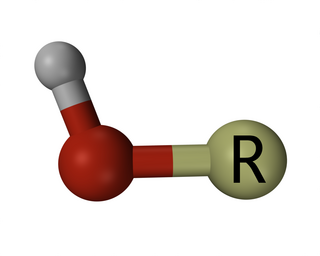
A hydroxy or hydroxyl group is a functional group with the chemical formula -OH and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy groups. Both the negatively charged anion OH−, called hydroxide, and the neutral radical ·OH, known as the hydroxyl radical, consist of an unbounded hydroxyl group.

Nucleotides are organic molecules consisting of a nucleoside and a phosphate. They serve as monomeric units of the nucleic acid polymers deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both of which are essential biomolecules within all life-forms on Earth. Nucleotides are obtained in the diet and are also synthesized from common nutrients by the liver.

Organic chemistry is a branch of chemistry that studies the structure, properties and reactions of organic compounds, which contain carbon in covalent bonding. Study of structure determines their chemical composition and formula. Study of properties includes physical and chemical properties, and evaluation of chemical reactivity to understand their behavior. The study of organic reactions includes the chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical study.
In chemistry, a zwitterion, also called an inner salt, is a molecule that contains an equal number of positively- and negatively-charged functional groups. With amino acids, for example, in solution a chemical equilibrium will be established between the "parent" molecule and the zwitterion.
In polymer science, the backbone chain of a polymer is the longest series of covalently bonded atoms that together create the continuous chain of the molecule. This science is subdivided into the study of organic polymers, which consist of a carbon backbone, and inorganic polymers which have backbones containing only main group elements.
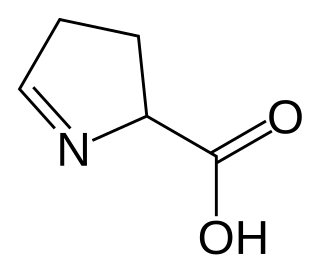
In chemistry, an imino acid is any molecule that contains both imine (>C=NH) and carboxyl functional groups.
Oh, OH or Oh! is an interjection, proclaiming surprise. It may refer to:

A biomolecule or biological molecule is a loosely used term for molecules present in organisms that are essential to one or more typically biological processes, such as cell division, morphogenesis, or development. Biomolecules include large macromolecules such as proteins, carbohydrates, lipids, and nucleic acids, as well as small molecules such as primary metabolites, secondary metabolites and natural products. A more general name for this class of material is biological materials. Biomolecules are an important element of living organisms, those biomolecules are often endogenous, produced within the organism but organisms usually need exogenous biomolecules, for example certain nutrients, to survive.
A polyamide is a polymer with repeating units linked by amide bonds.
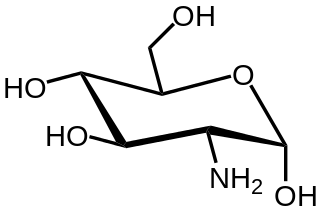
In organic chemistry, an amino sugar is a sugar molecule in which a hydroxyl group has been replaced with an amine group. More than 60 amino sugars are known, with one of the most abundant being N-Acetyl-d-glucosamine, which is the main component of chitin.
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the amine group is bonded to another carboxylic group in a protein to make it a chain, but since the end amino acid of a protein is only connected at the carboxy- end, the remaining free amine group is called the N-terminus. By convention, peptide sequences are written N-terminus to C-terminus, left to right (in LTR writing systems). This correlates the translation direction to the text direction (because when a protein is translated from messenger RNA, it is created from N-terminus to C-terminus - amino acids are added to the carboxyl end).

The alpha carbon (Cα) in organic molecules refers to the first carbon atom that attaches to a functional group, such as a carbonyl. The second carbon atom is called the beta carbon (Cβ), and the system continues naming in alphabetical order with Greek letters.

The Petasis reaction is the multi-component reaction of an amine, a carbonyl, and a vinyl- or aryl-boronic acid to form substituted amines.
An amine is one of a group of compounds containing a nitrogen atom with a lone pair.
Amide group may refer to:
Carbamic acid, which might also be called aminoformic acid or aminocarboxylic acid, is the chemical compound with the formula H2NCOOH. It can be obtained by the reaction of ammonia NH3 and carbon dioxide CO2 at very low temperatures, which also yields an equal amount of ammonium carbamate. The compound is stable only up to about 250 K (−23 °C); at higher temperatures it decomposes into those two gases. The solid apparently consists of dimers, with the two molecules connected by hydrogen bonds between the two carboxyl groups –COOH.
Alkanolamines are chemical compounds that contain both hydroxyl (-OH) and amino (-NH2, -NHR, and -NR2) functional groups on an alkane backbone. The term alkanolamine is a broad class term that is sometimes used as a subclassification.








