
An acyl group is a moiety derived by the removal of one or more hydroxyl groups from an oxoacid, including inorganic acids. It contains a double-bonded oxygen atom and an alkyl group (R-C=O). In organic chemistry, the acyl group is usually derived from a carboxylic acid. Therefore, it has the formula RCO–, where R represents an alkyl group that is linked to the carbon atom of the group by a single bond. Although the term is almost always applied to organic compounds, acyl groups can in principle be derived from other types of acids such as sulfonic acids, phosphonic acids. In the most common arrangement, acyl groups are attached to a larger molecular fragment, in which case the carbon and oxygen atoms are linked by a double bond.
A nucleophilic substitution is a class of chemical reactions in which an electron-rich chemical species replaces a functional group within another electron-deficient molecule. The molecule that contains the electrophile and the leaving functional group is called the substrate.
A polyamide is a polymer with repeating units linked by amide bonds.
The Friedel–Crafts reactions are a set of reactions developed by Charles Friedel and James Crafts in 1877 to attach substituents to an aromatic ring. Friedel–Crafts reactions are of two main types: alkylation reactions and acylation reactions. Both proceed by electrophilic aromatic substitution.
In organic chemistry, an acyl chloride (or acid chloride) is an organic compound with the functional group -COCl. Their formula is usually written RCOCl, where R is a side chain. They are reactive derivatives of carboxylic acids. A specific example of an acyl chloride is acetyl chloride, CH3COCl. Acyl chlorides are the most important subset of acyl halides.
In chemistry, acylation is the process of adding an acyl group to a compound. The compound providing the acyl group is called the acylating agent.

An acyl halide is a chemical compound derived from an oxoacid by replacing a hydroxyl group with a halide group.

Aluminium chloride (AlCl3), also known as aluminium trichloride, describe compounds with the formula AlCl3(H2O)n (n = 0 or 6). They consist of aluminium and chlorine atoms in a 1:3 ratio, and one form also contains six waters of hydration. Both are white solids, but samples are often contaminated with iron(III) chloride, giving a yellow color.

Acetyl chloride (CH3COCl) is an acyl chloride derived from acetic acid. It belongs to the class of organic compounds called acyl halides. It is a colorless, corrosive, volatile liquid. Its formula is commonly abbreviated to AcCl.

Thionyl chloride is an inorganic compound with the chemical formula SOCl
2. It is a moderately volatile colourless liquid with an unpleasant acrid odour. Thionyl chloride is primarily used as a chlorinating reagent, with approximately 45,000 tonnes per year being produced during the early 1990s, but is occasionally also used as a solvent. It is toxic, reacts with water, and is also listed under the Chemical Weapons Convention as it may be used for the production of chemical weapons.

Hydrogen halides are diatomic, inorganic compounds that function as Arrhenius acids. The formula is HX where X is one of the halogens: fluorine, chlorine, bromine, iodine, or astatine. All known hydrogen halides are gasses at Standard Temperature and Pressure.

An organic acid anhydride is an acid anhydride that is an organic compound. An acid anhydride is a compound that has two acyl groups bonded to the same oxygen atom. A common type of organic acid anhydride is a carboxylic anhydride, where the parent acid is a carboxylic acid, the formula of the anhydride being (RC(O))2O. Symmetrical acid anhydrides of this type are named by replacing the word acid in the name of the parent carboxylic acid by the word anhydride. Thus, (CH3CO)2O is called acetic anhydride. Mixed (or unsymmetrical) acid anhydrides, such as acetic formic anhydride (see below), are known, whereby reaction occurs between two different carboxylic acids. Nomenclature of unsymmetrical acid anhydrides list the names of both of the reacted carboxylic acids before the word "anhydride" (for example, the dehydration reaction between benzoic acid and propanoic acid would yield "benzoic propanoic anhydride").
The Fries rearrangement, named for the German chemist Karl Theophil Fries, is a rearrangement reaction of a phenolic ester to a hydroxy aryl ketone by catalysis of Lewis acids.
Nucleophilic acyl substitution describe a class of substitution reactions involving nucleophiles and acyl compounds. In this type of reaction, a nucleophile – such as an alcohol, amine, or enolate – displaces the leaving group of an acyl derivative – such as an acid halide, anhydride, or ester. The resulting product is a carbonyl-containing compound in which the nucleophile has taken the place of the leaving group present in the original acyl derivative. Because acyl derivatives react with a wide variety of nucleophiles, and because the product can depend on the particular type of acyl derivative and nucleophile involved, nucleophilic acyl substitution reactions can be used to synthesize a variety of different products.
The Rosenmund reduction is a hydrogenation process in which an acyl chloride is selectively reduced to an aldehyde. The reaction was named after Karl Wilhelm Rosenmund, who first reported it in 1918.
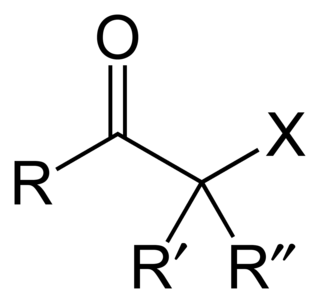
In organic chemistry, an α-haloketone is a functional group consisting of a ketone group or more generally a carbonyl group with an α-halogen substituent. α-haloketones are alkylating agents. Prominent α-haloketones include phenacyl bromide and chloroacetone.
Acetyl iodide is an organoiodine compound with the formula CH3COI. It is a colourless liquid. It is formally derived from acetic acid. Although far rarer in the laboratory than the related acetyl bromide and acetyl chloride, acetyl iodide is produced, transiently at least, on a far larger scale than any other acid halide. Specifically, it is generated by the carbonylation of methyl iodide in the Cativa and Monsanto processes that are the main industrial route to acetic acid. It is also an intermediate in the production of acetic anhydride from methyl acetate.

In organic chemistry, carbonyl reduction is the organic reduction of any carbonyl group by a reducing agent.

Imidoyl chlorides are organic compounds that contain the functional group RC(NR')Cl. A double bond exist between the R'N and the carbon centre. These compounds are analogues of acyl chloride. Imidoyl chlorides tend to be highly reactive and are more commonly found as intermediates in a wide variety of synthetic procedures. Such procedures include Gattermann aldehyde synthesis, Houben-Hoesch ketone synthesis, and the Beckmann rearrangement. Their chemistry is related to that of enamines and their tautomers when the α hydrogen is next to the C=N bond. Many chlorinated N-heterocycles are formally imidoyl chlorides, e.g. 2-chloropyridine, 2, 4, and 6-chloropyrimidines.
In organic chemistry, thioacyl chloride is a functional group of the type RC(S)Cl, where R is an organic substituent. Thioacyl chlorides are analogous to acid chlorides, but much rarer and less robust. The best studied is thiobenzoyl chloride, a purple oil first prepared by chlorination of dithiobenzoic acid with a combination of chlorine and thionyl chloride. A more modern preparation employs phosgene as the chlorinating agent, this also generates carbonyl sulfide as a by-product.










