
Sonic hedgehog is a protein encoded for by the SHH gene. The protein is named after the character Sonic the Hedgehog.
A coenocyte is a multinucleate cell which can result from multiple nuclear divisions without their accompanying cytokinesis, in contrast to a syncytium, which results from cellular aggregation followed by dissolution of the cell membranes inside the mass. The word syncytium in animal embryology is used to refer to the coenocytic blastoderm of invertebrates. A coenocytic cell is referred to as a coenobium, and most coenobia are composed of a distinct number of cells, often as a multiple of two.

Drosophila embryogenesis, the process by which Drosophila embryos form, is a favorite model system for genetics and developmental biology. The study of its embryogenesis unlocked the century-long puzzle of how development was controlled, creating the field of evolutionary developmental biology. The small size, short generation time, and large brood size make it ideal for genetic studies. Transparent embryos facilitate developmental studies. Drosophila melanogaster was introduced into the field of genetic experiments by Thomas Hunt Morgan in 1909.

A morphogen is a substance whose non-uniform distribution governs the pattern of tissue development in the process of morphogenesis or pattern formation, one of the core processes of developmental biology, establishing positions of the various specialized cell types within a tissue. More specifically, a morphogen is a signaling molecule that acts directly on cells to produce specific cellular responses depending on its local concentration.
Hox genes, a subset of homeobox genes, are a group of related genes that specify regions of the body plan of an embryo along the head-tail axis of animals. Hox proteins encode and specify the characteristics of 'position', ensuring that the correct structures form in the correct places of the body. For example, Hox genes in insects specify which appendages form on a segment, and Hox genes in vertebrates specify the types and shape of vertebrae that will form. In segmented animals, Hox proteins thus confer segmental or positional identity, but do not form the actual segments themselves.
Compartments can be simply defined as separate, different, adjacent cell populations, which upon juxtaposition, create a lineage boundary. This boundary prevents cell movement from cells from different lineages across this barrier, restricting them to their compartment. Subdivisions are established by morphogen gradients and maintained by local cell-cell interactions, providing functional units with domains of different regulatory genes, which give rise to distinct fates. Compartment boundaries are found across species. In the hindbrain of vertebrate embryos, rhobomeres are compartments of common lineage outlined by expression of Hox genes. In invertebrates, the wing imaginal disc of Drosophila provides an excellent model for the study of compartments. Although other tissues, such as the abdomen, and even other imaginal discs are compartmentalized, much of our understanding of key concepts and molecular mechanisms involved in compartment boundaries has been derived from experimentation in the wing disc of the fruit fly.

Krüppel is a gap gene in Drosophila melanogaster, located on the 2R chromosome, which encodes a zinc finger C2H2 transcription factor. Gap genes work together to establish the anterior-posterior segment patterning of the insect through regulation of the transcription factor encoding pair rule genes. These genes in turn regulate segment polarity genes. Krüppel means "cripple" in German, named for the crippled appearance of mutant larvae, who have failed to develop proper thoracic and anterior segments in the abdominal region. Mutants can also have abdominal mirror duplications.

A gap gene is a type of gene involved in the development of the segmented embryos of some arthropods. Gap genes are defined by the effect of a mutation in that gene, which causes the loss of contiguous body segments, resembling a gap in the normal body plan. Each gap gene, therefore, is necessary for the development of a section of the organism.
An asymmetric cell division produces two daughter cells with different cellular fates. This is in contrast to symmetric cell divisions which give rise to daughter cells of equivalent fates. Notably, stem cells divide asymmetrically to give rise to two distinct daughter cells: one copy of the original stem cell as well as a second daughter programmed to differentiate into a non-stem cell fate.
Decapentaplegic (Dpp) is a key morphogen involved in the development of the fruit fly Drosophila melanogaster and is the first validated secreted morphogen. It is known to be necessary for the correct patterning and development of the early Drosophila embryo and the fifteen imaginal discs, which are tissues that will become limbs and other organs and structures in the adult fly. It has also been suggested that Dpp plays a role in regulating the growth and size of tissues. Flies with mutations in decapentaplegic fail to form these structures correctly, hence the name. Dpp is the Drosophila homolog of the vertebrate bone morphogenetic proteins (BMPs), which are members of the TGF-β superfamily, a class of proteins that are often associated with their own specific signaling pathway. Studies of Dpp in Drosophila have led to greater understanding of the function and importance of their homologs in vertebrates like humans.
In the field of developmental biology, regional differentiation is the process by which different areas are identified in the development of the early embryo. The process by which the cells become specified differs between organisms.

The bicoid 3′-UTR regulatory element is an mRNA regulatory element that controls the gene expression of the bicoid protein in fruitfly Drosophila melanogaster.
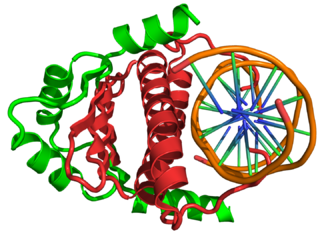
In the field of molecular biology, myocyte enhancer factor-2 (Mef2) proteins are a family of transcription factors which through control of gene expression are important regulators of cellular differentiation and consequently play a critical role in embryonic development. In adult organisms, Mef2 proteins mediate the stress response in some tissues. Mef2 proteins contain both MADS-box and Mef2 DNA-binding domains.

mRNA localization is a common mode of posttranscriptional regulation of gene expression that targets a protein to its site of function. Proteins are highly dependent on cellular environments for stability and function, therefore, mRNA localization signals are crucial for maintaining protein function. The Gurken localisation signal is an RNA regulatory element conserved across many species of Drosophila. The element consists of an RNA stem loop within the coding region of the messenger RNA that forms a signal for dynein-mediated Gurken mRNA transport to the dorsoanterior cap near the nucleus of the oocyte.

The French flag model is a conceptual definition of a morphogen, described by Lewis Wolpert in the 1960s. A morphogen is defined as a signaling molecule that acts directly on cells to produce specific cellular responses dependent on morphogen concentration. During early development, morphogen gradients generate different cell types in distinct spatial order. French flag patterning is often found in combination with others: vertebrate limb development is one of the many phenotypes exhibiting Turing overlapped with a complementary pattern.

Hematopoietically-expressed homeobox protein HHEX is a protein that in humans is encoded by the HHEX gene and also known as Proline Rich Homeodomain protein PRH.

Homeobox protein OTX2 is a protein that in humans is encoded by the OTX2 gene.
Maternal to zygotic transition is the stage in embryonic development during which development comes under the exclusive control of the zygotic genome rather than the maternal (egg) genome. The egg contains stored maternal genetic material mRNA which controls embryo development until the onset of MZT. After MZT the diploid embryo takes over genetic control. This requires both zygotic genome activation (ZGA) and degradation of maternal products. This process is important because it is the first time that the new embryonic genome is utilized and the paternal and maternal genomes are used in combination. The zygotic genome now drives embryo development.
Vasa is an RNA binding protein with an ATP-dependent RNA helicase that is a member of the DEAD box family of proteins. The vasa gene, is essential for germ cell development and was first identified in Drosophila melanogaster, but has since been found to be conserved in a variety of vertebrates and invertebrates including humans. The Vasa protein is found primarily in germ cells in embryos and adults, where it is involved in germ cell determination and function, as well as in multipotent stem cells, where its exact function is unknown.
Elizabeth Gavis is an American biologist who is the Damon B. Pfeiffer Professor of Life Sciences, at Princeton University. Davis served as the President of the North American Drosophila Board of Directors in 2011.













