
Boron nitride is a thermally and chemically resistant refractory compound of boron and nitrogen with the chemical formula BN. It exists in various crystalline forms that are isoelectronic to a similarly structured carbon lattice. The hexagonal form corresponding to graphite is the most stable and soft among BN polymorphs, and is therefore used as a lubricant and an additive to cosmetic products. The cubic variety analogous to diamond is called c-BN; it is softer than diamond, but its thermal and chemical stability is superior. The rare wurtzite BN modification is similar to lonsdaleite but slightly softer than the cubic form.

Boron is a chemical element with the symbol B and atomic number 5. Produced entirely by cosmic ray spallation and supernovae and not by stellar nucleosynthesis, it is a low-abundance element in the Solar System and in the Earth's crust. It constitutes about 0.001 percent by weight of Earth's crust. Boron is concentrated on Earth by the water-solubility of its more common naturally occurring compounds, the borate minerals. These are mined industrially as evaporites, such as borax and kernite. The largest known boron deposits are in Turkey, the largest producer of boron minerals.
A period 2 element is one of the chemical elements in the second row of the periodic table of the chemical elements. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behavior of the elements as their atomic number increases; a new row is started when chemical behavior begins to repeat, creating columns of elements with similar properties.
Nitrogen oxide may refer to a binary compound of oxygen and nitrogen, or a mixture of such compounds:
Sulfur trioxide (alternative spelling sulphur trioxide) is the chemical compound with the formula SO3, with a relatively narrow liquid range. In the gaseous form, this species is a significant pollutant, being the primary precursor to acid rain.
A trioxide is a compound with three oxygen atoms. For metals with the M2O3 formula there are several common structures. Al2O3, Cr2O3, Fe2O3, and V2O3 adopt the corundum structure. Many rare earth oxides adopt the "A-type rare earth structure" which is hexagonal. Several others plus indium oxide adopt the "C-type rare earth structure", also called "bixbyite", which is cubic and related to the fluorite structure.

Tungsten(VI) oxide, also known as tungsten trioxide or tungstic anhydride, WO3, is a chemical compound containing oxygen and the transition metal tungsten. It is obtained as an intermediate in the recovery of tungsten from its minerals. Tungsten ores are treated with alkalis to produce WO3. Further reaction with carbon or hydrogen gas reduces tungsten trioxide to the pure metal.
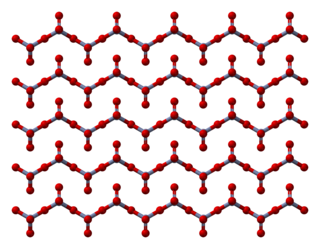
Chromium trioxide (also known as chromium(VI) oxide or chromic anhydride) is an inorganic compound with the formula CrO3. It is the acidic anhydride of chromic acid, and is sometimes marketed under the same name. This compound is a dark-purple solid under anhydrous conditions, bright orange when wet and which dissolves in water concomitant with hydrolysis. Millions of kilograms are produced annually, mainly for electroplating. Chromium trioxide is a powerful oxidiser and a carcinogen.

Boron trioxide (or diboron trioxide) is one of the oxides of boron. It is a white, glassy solid with the formula B2O3. It is almost always found as the vitreous (amorphous) form; however, it can be crystallized after extensive annealing (that is, under prolonged heat).

Arsenic trioxide, sold under the brand name Trisenox among others, is an inorganic compound and medication. As an industrial chemical, major uses include in the manufacture of wood preservatives, pesticides, and glass. As a medication it is used to treat a type of cancer known as acute promyelocytic leukemia. For this use it is given by injection into a vein.

Uranium trioxide (UO3), also called uranyl oxide, uranium(VI) oxide, and uranic oxide, is the hexavalent oxide of uranium. The solid may be obtained by heating uranyl nitrate to 400 °C. Its most commonly encountered polymorph, γ-UO3, is a yellow-orange powder.

Xenon trioxide is an unstable compound of xenon in its +6 oxidation state. It is a very powerful oxidizing agent, and liberates oxygen from water slowly, accelerated by exposure to sunlight. It is dangerously explosive upon contact with organic materials. When it detonates, it releases xenon and oxygen gas.
This is the list of extremely hazardous substances defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act. The list can be found as an appendix to 40 C.F.R. 355. Updates as of 2006 can be seen on the Federal Register, 71 FR 47121.

Boron sulfide is the chemical compound with the formula B2S3. This polymeric material that has been of interest as a component of "high-tech" glasses and as a reagent for preparing organosulfur compounds. Like the sulfides of silicon and phosphorus, B2S3 reacts with water, including atmospheric moisture to release H2S. Thus, samples must be handled under anhydrous conditions.

Rhenium trioxide or rhenium(VI) oxide is an inorganic compound with the formula ReO3. It is a red solid with a metallic lustre, which resembles copper in appearance. It is the only stable trioxide of the Group 7 elements (Mn, Tc, Re).

Boron suboxide (chemical formula B6O) is a solid compound with a structure built of eight icosahedra at the apexes of the rhombohedral unit cell. Each icosahedron is composed of twelve boron atoms. Two oxygen atoms are located in the interstices along the [111] rhombohedral direction. Due to its short interatomic bond lengths and strongly covalent character, B6O displays a range of outstanding physical and chemical properties such as great hardness (close to that of rhenium diboride and boron nitride), low mass density, high thermal conductivity, high chemical inertness, and excellent wear resistance.
Boron monoxide (B2O) is a chemical compound of boron and oxygen. Two experimental studies have proposed existence of diamond-like and graphite-like B2O, as for boron nitride and carbon solids. However, a later, systematic, experimental study of boron oxide phase diagram suggests that B2O is unstable. The instability of the graphite-like B2O phase was also predicted theoretically.

Dinitrogen trioxide is the chemical compound with the formula N2O3. This deep blue solid is one of the simple nitrogen oxides. It forms upon mixing equal parts of nitric oxide and nitrogen dioxide and cooling the mixture below −21 °C (−6 °F):

The Wright etch is a preferential etch for revealing defects in <100>- and <111>-oriented, p- and n-type silicon wafers used for making transistors, microprocessors, memories, and other components. Revealing, identifying, and remedying such defects is essential for progress along the path predicted by Moore's Law. It was developed by Margaret Wright Jenkins (1936--2018) in 1976 while working in research and development at Motorola Inc. in Phoenix, AZ. It was published in 1977. This etchant reveals clearly defined oxidation-induced stacking faults, dislocations, swirls and striations with minimum surface roughness or extraneous pitting. These defects are known causes of shorts and current leakage in finished semiconductor devices should they fall across isolated junctions. A relatively low etch rate at room temperature provides etch control. The long shelf life of this etchant allows the solution to be stored in large quantities.
Macor is the trademark for a machinable glass-ceramic developed and sold by Corning Inc. It is a white material that looks somewhat like porcelain. Macor is a good thermal insulator and is stable up to temperatures of 1000 °C, with very little thermal expansion or outgassing. It can be machined using standard metalworking tools.












