
An analgesic drug, also called simply an analgesic, pain reliever, or painkiller, is any member of the group of drugs used to achieve relief from pain. Analgesics are conceptually distinct from anesthetics, which temporarily reduce, and in some instances eliminate, sensation, although analgesia and anesthesia are neurophysiologically overlapping and thus various drugs have both analgesic and anesthetic effects.

Antipsychotics, also known as neuroleptics, are a class of psychotropic medication primarily used to manage psychosis, principally in schizophrenia but also in a range of other psychotic disorders. They are also the mainstay together with mood stabilizers in the treatment of bipolar disorder.

Malaria is a mosquito-borne infectious disease that affects humans and other vertebrates. Human malaria causes symptoms that typically include fever, fatigue, vomiting, and headaches. In severe cases, it can cause jaundice, seizures, coma, or death. Symptoms usually begin 10 to 15 days after being bitten by an infected Anopheles mosquito. If not properly treated, people may have recurrences of the disease months later. In those who have recently survived an infection, reinfection usually causes milder symptoms. This partial resistance disappears over months to years if the person has no continuing exposure to malaria.

A dietary supplement is a manufactured product intended to supplement one's diet by taking a pill, capsule, tablet, powder, or liquid. A supplement can provide nutrients either extracted from food sources or that are synthetic in order to increase the quantity of their consumption. The class of nutrient compounds includes vitamins, minerals, fiber, fatty acids, and amino acids. Dietary supplements can also contain substances that have not been confirmed as being essential to life, but are marketed as having a beneficial biological effect, such as plant pigments or polyphenols. Animals can also be a source of supplement ingredients, such as collagen from chickens or fish for example. These are also sold individually and in combination, and may be combined with nutrient ingredients. The European Commission has also established harmonized rules to help insure that food supplements are safe and appropriately labeled.
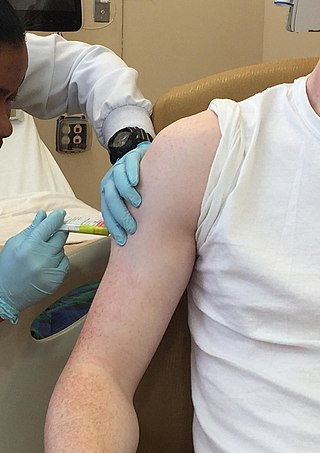
Clinical trials are prospective biomedical or behavioral research studies on human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments and known interventions that warrant further study and comparison. Clinical trials generate data on dosage, safety and efficacy. They are conducted only after they have received health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial—their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted.

Pharmacy is the science and practice of discovering, producing, preparing, dispensing, reviewing and monitoring medications, aiming to ensure the safe, effective, and affordable use of medicines. It is a miscellaneous science as it links health sciences with pharmaceutical sciences and natural sciences. The professional practice is becoming more clinically oriented as most of the drugs are now manufactured by pharmaceutical industries. Based on the setting, pharmacy practice is either classified as community or institutional pharmacy. Providing direct patient care in the community of institutional pharmacies is considered clinical pharmacy.

Piracetam is a drug marketed as a treatment for myoclonus. It is also used as a cognitive enhancer to improve memory, attention, and learning. Evidence to support its use is unclear, with some studies showing modest benefits in specific populations and others showing minimal or no benefit. Piracetam is sold as a medication in many European countries. Sale of piracetam is not illegal in the United States, although it is not regulated nor approved by the FDA so it is legally sold for research use only.

Pharmacogenomics is the study of the role of the genome in drug response. Its name reflects its combining of pharmacology and genomics. Pharmacogenomics analyzes how the genetic makeup of a patient affects their response to drugs. It deals with the influence of acquired and inherited genetic variation on drug response, by correlating DNA mutations with pharmacokinetic, pharmacodynamic, and/or immunogenic endpoints.
An adverse event (AE) is any untoward medical occurrence in a patient or clinical investigation subject administered a pharmaceutical product and which does not necessarily have a causal relationship with this treatment. An adverse event can therefore be any unfavourable and unintended sign, symptom, or disease temporally associated with the use of a medicinal (investigational) product, whether or not related to the medicinal (investigational) product.
The Health Improvement Network (THIN) is a large database of anonymised electronic medical records collected at primary care clinics throughout the UK. The THIN database is owned and managed by The Health Improvement Network Ltd in collaboration with In Practice Systems Ltd.

In fields such as epidemiology, social sciences, psychology and statistics, an observational study draws inferences from a sample to a population where the independent variable is not under the control of the researcher because of ethical concerns or logistical constraints. One common observational study is about the possible effect of a treatment on subjects, where the assignment of subjects into a treated group versus a control group is outside the control of the investigator. This is in contrast with experiments, such as randomized controlled trials, where each subject is randomly assigned to a treated group or a control group. Observational studies, for lacking an assignment mechanism, naturally present difficulties for inferential analysis.

Uppsala Monitoring Centre (UMC), located in Uppsala, Sweden, is the field name for the World Health Organization Collaborating Centre for International Drug Monitoring. UMC works by collecting, assessing and communicating information from member countries' national pharmacovigilance centres in regard to the benefits, harm, effectiveness and risks of drugs.
UpToDate, Inc. is a company in the Wolters Kluwer Health division of Wolters Kluwer, the main product of which is the eponymous UpToDate, a software system that is a point-of-care medical resource.
The Clinical Practice Research Datalink (CPRD) is an observational and interventional research service that operates as part of the UK Department of Health. It is jointly funded by the National Institute for Health and Care Research(NIHR) and the Medicines and Healthcare products Regulatory Agency (MHRA). CPRD is working closely with the extensive primary care, topic specific and comprehensive NIHR research networks and with NHS Digital.

The Center for Drug Evaluation and Research is a division of the U.S. Food and Drug Administration (FDA) that monitors most drugs as defined in the Food, Drug, and Cosmetic Act. Some biological products are also legally considered drugs, but they are covered by the Center for Biologics Evaluation and Research. The center reviews applications for brand name, generic, and over the counter pharmaceuticals, manages US current Good Manufacturing Practice (cGMP) regulations for pharmaceutical manufacturing, determines which medications require a medical prescription, monitors advertising of approved medications, and collects and analyzes safety data about pharmaceuticals that are already on the market.
Funding bias, also known as sponsorship bias, funding outcome bias, funding publication bias, and funding effect, refers to the tendency of a scientific study to support the interests of the study's financial sponsor. This phenomenon is recognized sufficiently that researchers undertake studies to examine bias in past published studies. Funding bias has been associated, in particular, with research into chemical toxicity, tobacco, and pharmaceutical drugs. It is an instance of experimenter's bias.
HIV prevention refers to practices that aim to prevent the spread of the human immunodeficiency virus (HIV). HIV prevention practices may be undertaken by individuals to protect their own health and the health of those in their community, or may be instituted by governments and community-based organizations as public health policies.
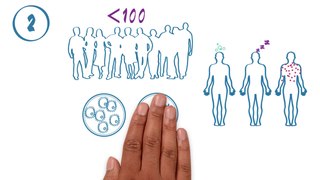
The phases of clinical research are the stages in which scientists conduct experiments with a health intervention to obtain sufficient evidence for a process considered effective as a medical treatment. For drug development, the clinical phases start with testing for drug safety in a few human subjects, then expand to many study participants to determine if the treatment is effective. Clinical research is conducted on drug candidates, vaccine candidates, new medical devices, and new diagnostic assays.
The distribution of medications has special drug safety and security considerations. Some drugs require cold chain management in their distribution.

Metiapine is a typical antipsychotic medication of the dibenzothiazepine group. There is scarce research on the safety and efficacy of metiapine in humans, though limited human trials exist.












