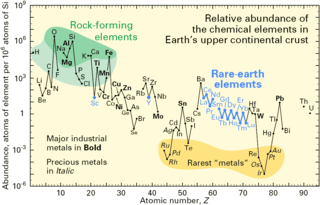
In physics, natural abundance (NA) refers to the abundance of isotopes of a chemical element as naturally found on a planet. The relative atomic mass of these isotopes is the atomic weight listed for the element in the periodic table. The abundance of an isotope varies from planet to planet, and even from place to place on the Earth, but remains relatively constant in time.

In nuclear physics, a decay product is the remaining nuclide left over from radioactive decay. Radioactive decay often proceeds via a sequence of steps. For example, 238U decays to 234Th which decays to 234mPa which decays, and so on, to 206Pb :

Uranium-238 is the most common isotope of uranium found in nature, with a relative abundance of 99%. Unlike uranium-235, it is non-fissile, which means it cannot sustain a chain reaction in a thermal-neutron reactor. However, it is fissionable by fast neutrons, and is fertile, meaning it can be transmuted to fissile plutonium-239. 238U cannot support a chain reaction because inelastic scattering reduces neutron energy below the range where fast fission of one or more next-generation nuclei is probable. Doppler broadening of 238U's neutron absorption resonances, increasing absorption as fuel temperature increases, is also an essential negative feedback mechanism for reactor control.

The mass number, also called atomic mass number or nucleon number, is the total number of protons and neutrons in an atomic nucleus. It is approximately equal to the atomic mass of the atom expressed in atomic mass units. Since protons and neutrons are both baryons, the mass number A is identical with the baryon number B of the nucleus. The mass number is different for each different isotope of a chemical element. Hence, the difference between the mass number and the atomic number Z gives the number of neutrons (N) in a given nucleus: N = A − Z.
In cell biology, bulk flow is the process by which proteins with a sorting signal travel to and from different cellular compartments. In other words, bulk transport is a type of transport which involves the transport of large amount of substance like lipid droplets and solid food particles across plasma membrane by utilising energy. Special processes are involved in the transport of such large quantities of materials, which include endocytosis and exocytosis.
Uranium–thorium dating, also called thorium-230 dating, uranium-series disequilibrium dating or uranium-series dating, is a radiometric dating technique established in the 1960s which has been used since the 1970s to determine the age of calcium carbonate materials such as speleothem or coral. Unlike other commonly used radiometric dating techniques such as rubidium–strontium or uranium–lead dating, the uranium-thorium technique does not measure accumulation of a stable end-member decay product. Instead, it calculates an age from the degree to which secular equilibrium has been restored between the radioactive isotope thorium-230 and its radioactive parent uranium-234 within a sample.
Uranium (92U) is a naturally occurring radioactive element that has no stable isotope. It has two primordial isotopes, uranium-238 and uranium-235, that have long half-lives and are found in appreciable quantity in the Earth's crust. The decay product uranium-234 is also found. Other isotopes such as uranium-233 have been produced in breeder reactors. In addition to isotopes found in nature or nuclear reactors, many isotopes with far shorter half-lives have been produced, ranging from 214U to 242U. The standard atomic weight of natural uranium is 238.02891(3).
Protactinium (91Pa) has no stable isotopes. The three naturally occurring isotopes allow a standard atomic weight to be given.
Natural nitrogen (7N) consists of two stable isotopes: the vast majority (99.6%) of naturally occurring nitrogen is nitrogen-14, with the remainder being nitrogen-15. Fourteen radioisotopes are also known, with atomic masses ranging from 10 to 25, along with one nuclear isomer, 11mN. All of these radioisotopes are short-lived, the longest-lived being nitrogen-13 with a half-life of 9.965 minutes. All of the others have half-lives below 7.15 seconds, with most of these being below 620 milliseconds. Most of the isotopes with atomic mass numbers below 14 decay to isotopes of carbon, while most of the isotopes with masses above 15 decay to isotopes of oxygen. The shortest-lived known isotope is nitrogen-10, with a half-life of about 200 yoctoseconds.
Americium (95Am) is an artificial element, and thus a standard atomic weight cannot be given. Like all artificial elements, it has no known stable isotopes. The first isotope to be synthesized was 241Am in 1944. The artificial element decays by ejecting alpha particles. Americium has an atomic number of 95.
Berkelium (97Bk) is an artificial element, and thus a standard atomic weight cannot be given. Like all artificial elements, it has no stable isotopes. The first isotope to be synthesized was 243Bk in 1949. There are 18 known radioisotopes, from 233Bk to 253Bk, and 6 nuclear isomers. The longest-lived isotope is 247Bk with a half-life of 1,380 years.

The Stowe Missal, which is, strictly speaking, a sacramentary rather than a missal, is an Irish illuminated manuscript written mainly in Latin with some Old Irish in the late eighth or early ninth century, probably after 792. In the mid-11th century it was annotated and some pages rewritten at Lorrha Monastery in County Tipperary, Ireland. Also known as the Lorrha Missal, it is known as the "Stowe" Missal as it once belonged to the Stowe manuscripts collection formed by George Nugent-Temple-Grenville, 1st Marquess of Buckingham at Stowe House. When the collection was bought by the nation in 1883, it and the other Irish manuscripts were handed over to the Royal Irish Academy in Dublin, where it remains, catalogued as MS D II 3. The cumdach or reliquary case which up to this point had survived together with the book was later transferred, with the rest of the Academy's collection of antiquities, to the National Museum of Ireland. The old story was that the manuscript and shrine left Ireland after about 1375, as they were collected on the Continent in the 18th century, but this appears to be incorrect, and they were found inside a stone wall at Lackeen Castle near Lorrha in the 18th century.
"Pie Jesu" is a text from the final couplet of the hymn, "Dies irae", and often included in musical settings of the Requiem Mass as a motet.
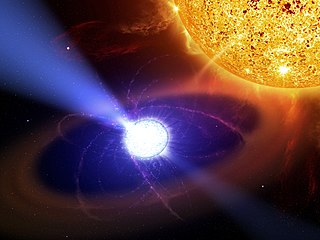
AE Aquarii is a cataclysmic variable binary star of the DQ Herculis type. Based upon parallax measurements, the system is located at a distance of about 280 light-years from the Earth. Because of its unique properties, this system has been subject to a number of scientific studies.

Nucleon pair breaking in fission has been an important topic in nuclear physics for decades. "Nucleon pair" refers to nucleon pairing effects which strongly influence the nuclear properties of a nuclide.
N-body units are a completely self-contained system of units used for N-body simulations of self-gravitating systems in astrophysics. In this system, the base physical units are chosen so that the total mass, M, the gravitational constant, G, and the virial radius, R, are normalized. The underlying assumption is that the system of N objects (stars) satisfies the virial theorem. The consequence of standard N-body units is that the velocity dispersion of the system, v, is and that the dynamical or crossing time, t, is . The use of standard N-body units was advocated by Michel Hénon in 1971. Early adopters of this system of units included H. Cohn in 1979 and D. Heggie and R. Mathieu in 1986. At the conference MODEST14 in 2014, D. Heggie proposed that the community abandon the name "N-body units" and replace it with the name "Hénon units" to commemorate the originator.
AE Andromedae is a luminous blue variable (LBV), a type of variable star. The star is one of the most luminous variables in M31, the Andromeda Galaxy.

NGC 605 is a lenticular galaxy in the constellation Andromeda, which is about 234 million light-years from the Milky Way. It was discovered on October 21, 1881 by the French astronomer Édouard Jean-Marie Stephan.

NGC 622 is a barred spiral galaxy located in the constellation Cetus about 234 million light-years from the Milky Way. It was discovered by British astronomer William Herschel in 1785.
The 1998 Holy Cross Crusaders football team was an American football team that represented the College of the Holy Cross during the 1998 NCAA Division I-AA football season. Holy Cross tied for last in the Patriot League.







