The molecular formula C19H14O6 (molar mass: 338.31 g/mol, exact mass: 338.0790 u) may refer to:
The molecular formula C19H14O6 (molar mass: 338.31 g/mol, exact mass: 338.0790 u) may refer to:
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and plus (+) and minus (−) signs. These are limited to a single typographic line of symbols, which may include subscripts and superscripts. A chemical formula is not a chemical name since it does not contain any words. Although a chemical formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. Chemical formulae can fully specify the structure of only the simplest of molecules and chemical substances, and are generally more limited in power than chemical names and structural formulae.
The molecular mass (m) is the mass of a given molecule: it is measured in daltons or atomic mass (Da or u). Different molecules of the same compound may have different molecular masses because they contain different isotopes of an element. The related quantity relative molecular mass, as defined by IUPAC, is the ratio of the mass of a molecule to the unified atomic mass unit (also known as the dalton) and is unitless. The molecular mass and relative molecular mass are distinct from but related to the molar mass. The molar mass is defined as the mass of a given substance divided by the amount of a substance and is expressed in g/mol. That makes the molar mass an average of many particles or molecules, and the molecular mass the mass of one specific particle or molecule. The molar mass is usually the more appropriate figure when dealing with macroscopic (weigh-able) quantities of a substance.
In chemistry, the molar mass of a chemical compound is defined as the ratio between the mass and the amount of substance of any sample of said compound. The molar mass is a bulk, not molecular, property of a substance. The molar mass is an average of many instances of the compound, which often vary in mass due to the presence of isotopes. Most commonly, the molar mass is computed from the standard atomic weights and is thus a terrestrial average and a function of the relative abundance of the isotopes of the constituent atoms on Earth. The molar mass is appropriate for converting between the mass of a substance and the amount of a substance for bulk quantities.
The molecular formula C21H23NO5 (molar mass: 369.41 g/mol, exact mass: 369.1576 u) may refer to:
The molecular formula C6H8O7 (molar mass: 192.12 g/mol, exact mass: 192.0270 u) may refer to:
The molecular formula BaSO4 (molar mass: 233.39 g/mol, exact mass: 233.8570 u) may refer to:
The molecular formula C2H6O (molar mass: 46.07 g/mol, exact mass: 46.0419 u) may refer to:
The molecular formula C4H10 (molar mass: 58.12 g/mol, exact mass: 58.0783 u) may refer to:
The molecular formula C6H10O7 (molar mass: 194.14 g/mol, exact mass: 194.0427 u) may refer to:
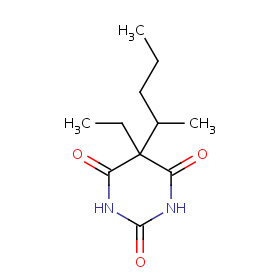
The molecular formula C11H18N2O3 (molar mass: 226.27 g/mol) may be referred as:
The molecular formula C14H12O3 (molar mass: 228.25 g/mol, exact mass: 228.078644 u) may refer to:
The molecular formula C21H22O4 (molar mass : 338.39 g/mol, exact mass : 338.151809 u) may refer to:
The molecular formula C19H30O5 (molar mass: 338.43 g/mol) may refer to:
The molecular formula C20H26N4O (molar mass: 338.45 g/mol, exact mass: 338.2107 u) may refer to:
The molecular formula C12H18BrNO2 (molar mass: 288.18 g/mol, exact mass: 287.0521 u) may refer to:
The molecular formula C20H18O5 (molar mass : 338.35 g/mol, exact mass : 338.115424 u) may refer to:
The molecular formula C19H19NO4 (molar mass: 325.35 g/mol, exact mass: 325.131408 u) may refer to:
The molecular formula C20H22N2O3 (molar mass: 338.400 g/mol, exact mass: 338.1630 u) may refer to:
The molecular formula C21H26N2O2 (molar mass: 338.44 g/mol) may refer to:
The molecular formula C17H18F3N3O3 (molar mass: 369.338 g/mol) may refer to: