Amyl nitrite is a chemical compound with the formula C5H11ONO. A variety of isomers are known, but they all feature an amyl group attached to the nitrite functional group. The alkyl group is unreactive and the chemical and biological properties are mainly due to the nitrite group. Like other alkyl nitrites, amyl nitrite is bioactive in mammals, being a vasodilator, which is the basis of its use as a prescription medicine. As an inhalant, it also has a psychoactive effect, which has led to its recreational use with its smell being described as that of old socks or dirty feet. It is also referred to as banapple gas.

An oxime is a chemical compound belonging to the imines, with the general formula RR'C=NOH, where R is an organic side-chain and R' may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted oximes form a closely related family of compounds. Amidoximes are oximes of amides with general structure R1C(=NOH)NR2R3.

Nitrous acid (molecular formula HNO2) is a weak and monobasic acid known only in solution and in the form of nitrite (NO−
2) salts. Nitrous acid is used to make diazonium salts from amines. The resulting diazonium salts are reagents in azo coupling reactions to give azo dyes.

The nitrite ion, which has the chemical formula NO−
2, is a symmetric anion with equal N–O bond lengths. Upon protonation, the unstable weak acid nitrous acid is produced. Nitrite can be oxidized or reduced, with the product somewhat dependent on the oxidizing/reducing agent and its strength. The nitrite ion is an ambidentate ligand, and is known to bond to metal centers in at least five different ways. Nitrite is also important in biochemistry as a source of the potent vasodilator nitric oxide. In organic chemistry the NO−
2 group is present in nitrous acid esters and nitro compounds. Nitrite is also used in the food production industry for curing meat.

Amyl nitrate is the chemical compound with the formula CH3(CH2)4ONO2. This molecule consists of the 5-carbon amyl group attached to a nitrate functional group. It is the ester of amyl alcohol and nitric acid.

Sodium nitrite is an inorganic compound with the chemical formula NaNO2. It is a white to slightly yellowish crystalline powder that is very soluble in water and is hygroscopic. It is a useful precursor to a variety of organic compounds, such as pharmaceuticals, dyes, and pesticides, but it is probably best known as a food additive used in processed meats and (in some countries) in fish products.

Nitro compounds are organic compounds that contain one or more nitro functional groups (−NO2). The nitro group is one of the most common explosophores (functional group that makes a compound explosive) used globally. The nitro group is also strongly electron-withdrawing. Because of this property, C−H bonds alpha (adjacent) to the nitro group can be acidic. For similar reasons, the presence of nitro groups in aromatic compounds retards electrophilic aromatic substitution but facilitates nucleophilic aromatic substitution. Nitro groups are rarely found in nature, being almost invariably produced by nitration reactions starting with nitric acid.

Nitrosamines are chemical compounds of the chemical structure R1N(–R2)–N=O, that is, a nitroso group bonded to an amine. Most nitrosamines are carcinogenic.
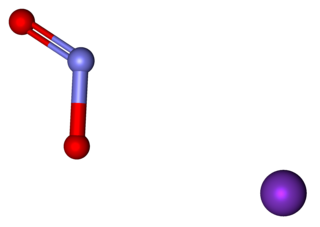
Potassium nitrite (distinct from potassium nitrate) is the inorganic compound with the chemical formula KNO2. It is an ionic salt of potassium ions K+ and nitrite ions NO2−, which forms a white or slightly yellow, hygroscopic crystalline powder that is soluble in water.
The Griess test is an analytical chemistry test which detects the presence of nitrite ion in solution. One of its most important uses is the determination of nitrite in drinking water. The test has also been widely used for the detection of trace explosives containing nitro groups. It is usually necessary to release the nitrite ion from the organic explosive before analysis. This is normally done by alkaline hydrolysis. The Griess diazotization reaction on which the Griess reagent relies was first described in 1858 by Peter Griess.
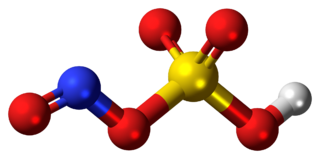
Nitrosylsulfuric acid is the chemical compound with the formula NOHSO4. It is a colourless solid that is used industrially in the production of caprolactam, and was formerly part of the lead chamber process for producing sulfuric acid. The compound is the mixed anhydride of sulfuric acid and nitrous acid.
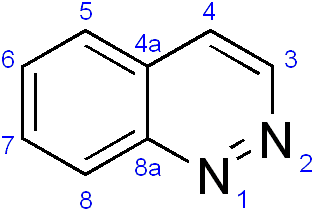
Cinnoline is an aromatic heterocyclic compound with the formula C8H6N2. It is isomeric with other naphthyridines including quinoxaline, phthalazine and quinazoline.

Nitroso refers to a functional group in organic chemistry which has the NO group attached to an organic moiety. As such, various nitroso groups can be categorized as C-nitroso compounds (e.g., nitrosoalkanes; R−N=O), S-nitroso compounds (nitrosothiols; RS−N=O), N-nitroso compounds (e.g., nitrosamines, R1N(−R2)−N=O), and O-nitroso compounds (alkyl nitrites; RO−N=O).
The Bamberger triazine synthesis in organic chemistry is a classic organic synthesis of a triazine first reported by Eugen Bamberger in 1892.

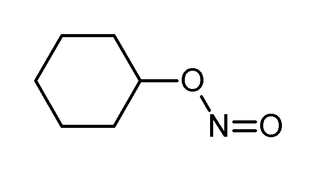
Alkyl nitrites are a group of chemical compounds based upon the molecular structure R-ONO. Formally they are alkyl esters of nitrous acid. They are distinct from nitro compounds (R-NO2).

Pipecolic acidemia, is a very rare autosomal recessive metabolic disorder that is caused by a peroxisomal defect.

Pipecolic acid is a small organic molecule which accumulates in pipecolic acidemia. It is a carboxylic acid of piperidine.

Castanospermine is an indolizidine alkaloid first isolated from the seeds of Castanospermum australe. It is a potent inhibitor of some glucosidase enzymes and has antiviral activity in vitro and in mouse models.

2-Iodobenzoic acid, or o-iodobenzoic acid, is an organic compound with the formula IC6H4COOH. The synthesis of 2-iodobenzoic acid via the diazotization of anthranilic acid is commonly performed in university organic chemistry labs. One of its most common uses is as a precursor for the preparation of IBX and Dess–Martin periodinane, both used as mild oxidants.