See also
| This set index page lists chemical structure articles associated with the same molecular formula. If an internal link led you here, you may wish to change the link to point directly to the intended article. |
The molecular formula C6H4O3 may refer to:
| This set index page lists chemical structure articles associated with the same molecular formula. If an internal link led you here, you may wish to change the link to point directly to the intended article. |
The cumene process is an industrial process for synthesizing phenol and acetone from benzene and propylene. The term stems from cumene, the intermediate material during the process. It was invented by Heinrich Hock in 1944 and independently by R. Ūdris and P. Sergeyev in 1942 (USSR).

Carotenoids, also called tetraterpenoids, are organic pigments that are produced by plants and algae, as well as several bacteria and fungi. Carotenoids give the characteristic color to carrots, corn, canaries, and daffodils, as well as egg yolks, rutabagas, buttercups, and bananas. Carotenoids can be produced from fats and other basic organic metabolic building blocks by all these organisms. The only animals known to produce carotenoids are aphids and spider mites, which acquired the ability and genes from fungi or it is produced by endosymbiotic bacteria in whiteflies. Carotenoids from the diet are stored in the fatty tissues of animals, and exclusively carnivorous animals obtain the compounds from animal fat.
The quinones are a class of organic compounds that are formally "derived from aromatic compounds [such as benzene or naphthalene] by conversion of an even number of –CH= groups into –C(=O)– groups with any necessary rearrangement of double bonds", resulting in "a fully conjugated cyclic dione structure". The class includes some heterocyclic compounds.
The Controlled Drugs and Substances Act is Canada's federal drug control statute. Passed in 1996 under Prime Minister Jean Chrétien's government, it repeals the Narcotic Control Act and Parts III and IV of the Food and Drugs Act; and establishes eight Schedules of controlled substances and two Classes of precursors. It provides that "The Governor in Council may, by order, amend any of Schedules I to VIII by adding to them or deleting from them any item or portion of an item, where the Governor in Council deems the amendment to be necessary in the public interest."

1,4-Benzoquinone, commonly known as para-quinone, is a chemical compound with the formula C6H4O2. In a pure state, it forms bright-yellow crystals with a characteristic irritating odor, resembling that of chlorine, bleach, and hot plastic or formaldehyde. This six-membered ring compound is the oxidized derivative of 1,4-hydroquinone. The molecule is multifunctional: it exhibits properties of a ketone, forming an oxime; an oxidant, forming the dihydroxy derivative; and an alkene, undergoing addition reactions, especially those typical for α,β-unsaturated ketones. 1,4-Benzoquinone is sensitive toward both strong mineral acids and alkali, which cause condensation and decomposition of the compound.
1,2-Benzoquinone, also called ortho-benzoquinone, is an organic compound with formula C6H4O2. It is one of the two isomers of quinone, the other being 1,4-benzoquinone. It is a red volatile solid that is soluble in water and ethyl ether. It is rarely encountered because of its instability, but it is of fundamental interest as the parent ortho-quinone, of which many analogues are known.

Benzoquinone (C6H4O2) is a quinone with a single benzene ring, of which there are only two:
Oppenauer oxidation, named after Rupert Viktor Oppenauer, is a gentle method for selectively oxidizing secondary alcohols to ketones.
3β-Hydroxysteroid dehydrogenase/Δ5-4 isomerase (3β-HSD) is an enzyme that catalyzes the biosynthesis of the steroid progesterone from pregnenolone, 17α-hydroxyprogesterone from 17α-hydroxypregnenolone, and androstenedione from dehydroepiandrosterone (DHEA) in the adrenal gland. It is the only enzyme in the adrenal pathway of corticosteroid synthesis that is not a member of the cytochrome P450 family. It is also present in other steroid-producing tissues, including the ovary, testis and placenta. In humans, there are two 3β-HSD isozymes encoded by the HSD3B1 and HSD3B2 genes.
Mequinol, MeHQ or 4-methoxyphenol, is a phenol used in dermatology and organic chemistry.
In enzymology, a 3-demethylubiquinone-9 3-O-methyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a vitamin-K-epoxide reductase (warfarin-insensitive) is an enzyme that catalyzes the chemical reaction
In enzymology, a 2-hydroxy-1,4-benzoquinone reductase (EC 1.6.5.7) is an enzyme that catalyzes the chemical reaction
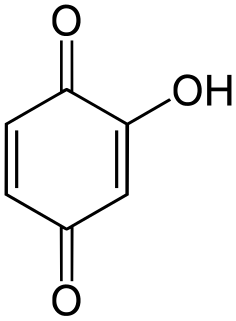
Hydroxyquinone often refers to a hydroxybenzoquinone, any organic compound with formula C
6H
4O
3 which can be viewed as a derivative of a benzoquinone through replacement of one hydrogen atom (H) by a hydroxyl group (-OH). When unqualified, the terms usually mean specifically the compound 2-hydroxy-1,4-benzoquinone, derived from 1,4-benzoquinone or para-benzoquinone.
A hydroxybenzoquinone is any of several organic compounds that can be viewed as derivatives of a benzoquinone through replacement of one hydrogen atom (H) by a hydroxyl group (-OH).
2,5-Dihydroxy-1,4-benzoquinone or 2,5-dihydroxy-para-benzoquinone is an organic compound with formula C
6H
4O
4, formally derived from 1,4-benzoquinone by replacing two hydrogen atoms with hydroxyl (OH) groups. It is one of seven dihydroxybenzoquinone isomers. It is a yellow solid with planar molecules that exhibits ferroelectric properties.

Hydroxy-1,4-benzoquinone, also called hydroxy-para-benzoquinone, is an organic compound with formula C
6H
4O
3, formally derived from 1,4-Benzoquinone by replacing one hydrogen atom with a hydroxyl (OH) group. It is one of three hydroxybenzoquinone isomers and one of the simplest hydroxyquinones.
The molecular formula C6H4O4 may refer to:

2,6-Dimethoxybenzoquinone (2,6-DMBQ) is a benzoquinone, a chemical compound found in Rauvolfia vomitoria and in Tibouchina pulchra.
4-nitrocatechol 4-monooxygenase (EC 1.14.13.166) is an enzyme with systematic name 4-nitrocatechol,NAD(P)H:oxygen 4-oxidoreductase (4-hydroxylating, nitrite-forming). This enzyme catalyses the following chemical reaction