The quinones are a class of organic compounds that are formally "derived from aromatic compounds [such as benzene or naphthalene] by conversion of an even number of –CH= groups into –C(=O)– groups with any necessary rearrangement of double bonds", resulting in "a fully conjugated cyclic dione structure". The class includes some heterocyclic compounds.
Dihydroxybenzenes are organic chemical compounds in which two hydroxyl groups are substituted onto a benzene ring. These aromatic compounds are classed as phenols. There are three isomer: 1,2-dihydroxybenzene is commonly known as catechol, 1,3-dihydroxybenzene is commonly known as resorcinol, and 1,4-dihydroxybenzene is commonly known as hydroquinone.
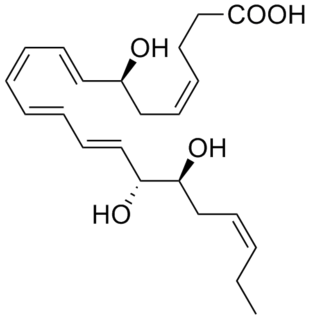
Resolvins are metabolic byproducts of omega-3 fatty acids, primarily eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), as well as docosapentaenoic acid (DPA) and clupanodonic acid. As autacoids similar to hormones acting on local tissues, resolvins are under preliminary research for their involvement in promoting restoration of normal cellular function following the inflammation that occurs after tissue injury. Resolvins belong to a class of polyunsaturated fatty acid (PUFA) metabolites termed specialized proresolving mediators (SPMs).
Arene substitution patterns are part of organic chemistry IUPAC nomenclature and pinpoint the position of substituents other than hydrogen in relation to each other on an aromatic hydrocarbon.

1,4-Benzoquinone, commonly known as para-quinone, is a chemical compound with the formula C6H4O2. In a pure state, it forms bright-yellow crystals with a characteristic irritating odor, resembling that of chlorine, bleach, and hot plastic or formaldehyde. This six-membered ring compound is the oxidized derivative of 1,4-hydroquinone. The molecule is multifunctional: it exhibits properties of a ketone, forming an oxime; an oxidant, forming the dihydroxy derivative; and an alkene, undergoing addition reactions, especially those typical for α,β-unsaturated ketones. 1,4-Benzoquinone is sensitive toward both strong mineral acids and alkali, which cause condensation and decomposition of the compound.
1,2-Benzoquinone, also called ortho-benzoquinone, is an organic compound with formula C6H4O2. It is one of the two isomers of quinone, the other being 1,4-benzoquinone. It is a red volatile solid that is soluble in water and ethyl ether. It is rarely encountered because of its instability, but it is of fundamental interest as the parent ortho-quinone, of which many analogues are known.

The Dakin oxidation is an organic redox reaction in which an ortho- or para-hydroxylated phenyl aldehyde or ketone reacts with hydrogen peroxide in base to form a benzenediol and a carboxylate. Overall, the carbonyl group is oxidized, and the hydrogen peroxide is reduced.

Phenanthreoids are chemical compounds formed with a phenanthrene backbone. Those compound are naturally occurring in plants, although they can also be synthetized.
In enzymology, a 2-hydroxy-1,4-benzoquinone reductase (EC 1.6.5.7) is an enzyme that catalyzes the chemical reaction

2,3,5,6,7,8-Hexahydroxy-1,4-naphthalenedione, also called hexahydroxynaphthoquinone or spinochrome E, is an organic compound with formula C
10H
6O
8. It is formally derived from naphthoquinone (1,4-naphtalenedione) through replacement of all six hydrogen atoms by hydroxyl (OH) groups. The numerical prefixes "2,3,5,6,7,8" are superfluous, since there is no other hexahydroxy derivative of 1,4-naphthoquinone.
A dihydroxyanthraquinone is any of several isomeric organic compounds with formula C
14H
8O
4, formally derived from 9,10-anthraquinone by replacing two hydrogen atoms by hydroxyl groups. Dihyroxyantraquinones have been studied since the early 1900s, and include some compounds of historical and current importance. The isomers differ in the position of the hydroxyl groups, and of the carbonyl oxygens (=O) of the underlying anthraquinone.
A hydroxynaphthoquinone is any of several organic compounds that can be viewed as derivatives of a naphthoquinone through replacement of one hydrogen atom (H) by a hydroxyl group (-OH).
A dihydroxynaphthoquinone is any of several organic compounds that can be viewed as derivatives of naphthoquinone through replacement of two hydrogen atoms (H) by hydroxyl groups (OH).

Naphthazarin, often called 5,8-dihydroxy-1,4-naphthoquinone or 5,8-dihydroxy-1,4-naphthalenedione (IUPAC), is a naturally occurring organic compound with formula C
10H
6O
4, formally derived from 1,4-naphthoquinone through replacement of two hydrogen atoms by hydroxyl (OH) groups. It is thus one of many dihydroxynaphthoquinone structural isomers.
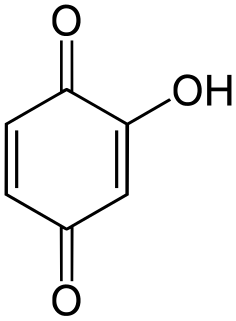
Hydroxyquinone often refers to a hydroxybenzoquinone, any organic compound with formula C
6H
4O
3 which can be viewed as a derivative of a benzoquinone through replacement of one hydrogen atom (H) by a hydroxyl group (-OH). When unqualified, the terms usually mean specifically the compound 2-hydroxy-1,4-benzoquinone, derived from 1,4-benzoquinone or para-benzoquinone.
A hydroxyanthraquinone (formula: C14H9O2(OH)) is any of several organic compounds that can be viewed as derivatives of an anthraquinone through replacement of one hydrogen atom (H) by a hydroxyl group (-OH). The IUPAC nomenclature recommends hydroxyanthracenedione.

Hydroxy-1,4-benzoquinone, also called hydroxy-para-benzoquinone, is an organic compound with formula C
6H
4O
3, formally derived from 1,4-Benzoquinone by replacing one hydrogen atom with a hydroxyl (OH) group. It is one of three hydroxybenzoquinone isomers and one of the simplest hydroxyquinones.
The molecular formula C6H4O4 may refer to:
The molecular formula C6H4O5 may refer to: