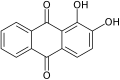
The quinones are a class of organic compounds that are formally "derived from aromatic compounds [such as benzene or naphthalene] by conversion of an even number of –CH= groups into –C(=O)– groups with any necessary rearrangement of double bonds, resulting in "a fully conjugated cyclic dione structure". The archetypical member of the class is 1,4-benzoquinone or cyclohexadienedione, often called simply "quinone". Other important examples are 1,2-benzoquinone (ortho-quinone), 1,4-naphthoquinone and 9,10-anthraquinone.

In organic chemistry, a dicarbonyl is a molecule containing two carbonyl groups. Although this term could refer to any organic compound containing two carbonyl groups, it is used more specifically to describe molecules in which both carbonyls are in close enough proximity that their reactivity is changed, such as 1,2-, 1,3-, and 1,4-dicarbonyls. Their properties often differ from those of monocarbonyls, and so they are usually considered functional groups of their own. These compounds can have symmetrical or unsymmetrical substituents on each carbonyl, and may also be functionally symmetrical or unsymmetrical.

Anthraquinone, also called anthracenedione or dioxoanthracene, is an aromatic organic compound with formula C
14H
8O
2. Isomers include various quinone derivatives. The term anthraquinone however refers to the isomer, 9,10-anthraquinone wherein the keto groups are located on the central ring. It is a building block of many dyes and is used in bleaching pulp for papermaking. It is a yellow, highly crystalline solid, poorly soluble in water but soluble in hot organic solvents. It is almost completely insoluble in ethanol near room temperature but 2.25 g will dissolve in 100 g of boiling ethanol. It is found in nature as the rare mineral hoelite.

1,4-Benzoquinone, commonly known as para-quinone, is a chemical compound with the formula C6H4O2. In a pure state, it forms bright-yellow crystals with a characteristic irritating odor, resembling that of chlorine, bleach, and hot plastic or formaldehyde. This six-membered ring compound is the oxidized derivative of 1,4-hydroquinone. The molecule is multifunctional: it exhibits properties of a ketone, being able to form oximes; an oxidant, forming the dihydroxy derivative; and an alkene, undergoing addition reactions, especially those typical for α,β-unsaturated ketones. 1,4-Benzoquinone is sensitive toward both strong mineral acids and alkali, which cause condensation and decomposition of the compound.
1,2-Benzoquinone, also called ortho-benzoquinone, is an organic compound with formula C6H4O2. It is one of the two isomers of quinone, the other being 1,4-benzoquinone. It is a red volatile solid that is soluble in water and ethyl ether. It is rarely encountered because of its instability, but it is of fundamental interest as the parent compound of many derivatives which are known.

Juglone, also called 5-hydroxy-1,4-naphthalenedione (IUPAC) is an organic compound with the molecular formula C10H6O3. In the food industry, juglone is also known as C.I. Natural Brown 7 and C.I. 75500. It is insoluble in benzene but soluble in dioxane, from which it crystallizes as yellow needles. It is an isomer of lawsone, which is the active dye compound in the henna leaf.

Spinochrome E, 2,3,5,6,7,8-Hexahydroxy-1,4-naphthalenedione, also called hexahydroxynaphthoquinone is a polyhydroxylated 1,4-naphthoquinones, pigments found in sea urchin shell ("test"), spine, gonads, coelomic fluid, and eggs, of sea urchin commonly known as spinochromes. These natural phenolic compounds are quinones that are known to have pharmacological properties. The several hydroxyl groups are appropriate for free-radical scavenging, which diminishes ROS and prevents redox imbalance. Mechanisms are described such as scavenging of reactive oxygen species (ROS), interaction with lipid peroxide radicals, chelation of metal ions, inhibition of lipid peroxidation and regulation of the cell redox potential.
A dihydroxyanthraquinone is any of several isomeric organic compounds with formula C
14H
8O
4, formally derived from 9,10-anthraquinone by replacing two hydrogen atoms by hydroxyl groups. Dihyroxyantraquinones have been studied since the early 1900s, and include some compounds of historical and current importance. The isomers differ in the position of the hydroxyl groups, and of the carbonyl oxygens (=O) of the underlying anthraquinone.
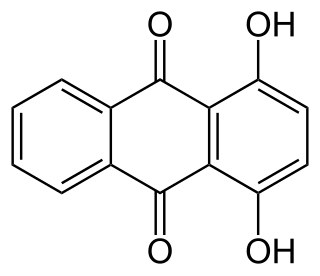
1,4-Dihydroxyanthraquinone, also called quinizarin or Solvent Orange 86, is an organic compound derived from anthroquinone. Quinizarin is an orange or red-brown crystalline powder. It is formally derived from anthraquinone by replacement of two hydrogen atoms by hydroxyl (OH) groups. It is one of ten dihydroxyanthraquinone isomers and occurs in small amounts in the root of the madder plant, Rubia tinctorum.
A hydroxynaphthoquinone is any of several organic compounds that can be viewed as derivatives of a naphthoquinone through replacement of one hydrogen atom (H) by a hydroxyl group (-OH).
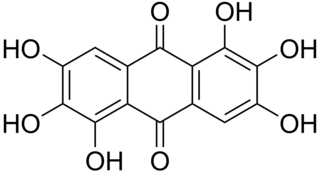
Rufigallol or 1,2,3,5,6,7-hexahydroxy-9,10-anthraquinone is an organic compound with formula C
14O
8H
8, which can be viewed as a derivative of anthraquinone through the replacement of six hydrogen atoms (H) by hydroxyl groups (OH).

2,3,5,6,8-Pentahydroxy-1,4-naphthalenedione, also called 2,3,5,6,8-pentahydroxy-1,4-naphthoquinone or spinochrome D, is an organic compound with formula C
10H
6O
5, formally derived from 1,4-naphthoquinone through the replacement of five hydrogen atoms by hydroxyl (OH) groups.
A dihydroxynaphthoquinone is any of several organic compounds that can be viewed as derivatives of naphthoquinone through replacement of two hydrogen atoms (H) by hydroxyl groups (OH).
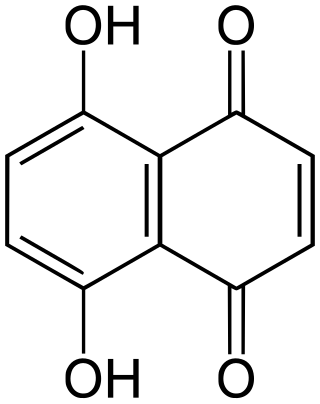
Naphthazarin, often called 5,8-dihydroxy-1,4-naphthoquinone or 5,8-dihydroxy-1,4-naphthalenedione (IUPAC), is a naturally occurring organic compound with formula C
10H
6O
4, formally derived from 1,4-naphthoquinone through replacement of two hydrogen atoms by hydroxyl (OH) groups. It is thus one of many dihydroxynaphthoquinone structural isomers.

A hydroxyanthraquinone (formula: C14H7O2(OH)) is any of several organic compounds that can be viewed as derivatives of an anthraquinone through replacement of one hydrogen atom (H) by a hydroxyl group (-OH).
A hydroxybenzoquinone is any of several organic compounds that can be viewed as derivatives of a benzoquinone through replacement of one hydrogen atom (H) by a hydroxyl group (-OH).
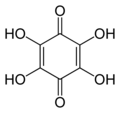
2,5-Dihydroxy-1,4-benzoquinone or 2,5-dihydroxy-para-benzoquinone is an organic compound with formula C
6H
4O
4, formally derived from 1,4-benzoquinone by replacing two hydrogen atoms with hydroxyl (OH) groups. It is one of seven dihydroxybenzoquinone isomers. It is a yellow solid with planar molecules that exhibits ferroelectric properties.

Hydroxy-1,4-benzoquinone, also called hydroxy-para-benzoquinone, is an organic compound with formula C
6H
4O
3, formally derived from 1,4-Benzoquinone by replacing one hydrogen atom with a hydroxyl (OH) group. It is one of three hydroxybenzoquinone isomers and one of the simplest hydroxyquinones.

Naphthoquinones constitute a class of organic compounds structurally related to naphthalene. Two isomers are common for the parent naphthoquinones:

1,4-Naphthoquinone or para-naphthoquinone is a quinone derived from naphthalene. It forms volatile yellow triclinic crystals and has a sharp odor similar to benzoquinone. It is almost insoluble in cold water, slightly soluble in petroleum ether, and more soluble in polar organic solvents. In alkaline solutions it produces a reddish-brown color. Vitamin K is a derivative of 1,4-naphthoquinone. It is a planar molecule with one aromatic ring fused to a quinone subunit. It is an isomer of 1,2-naphthoquinone.