
Acridine is an organic compound and a nitrogen heterocycle with the formula C13H9N. Acridines are substituted derivatives of the parent ring. It is a planar molecule that is structurally related to anthracene with one of the central CH groups replaced by nitrogen. Like the related molecule pyridine and quinoline, acridine is mildly basic. It is an almost colorless solid. There are no commercial applications of acridines but at one time acridine dyes were popular. It crystallizes in needles.

Carotenoids, also called tetraterpenoids, are organic pigments that are produced by plants and algae, as well as several bacteria and fungi. Carotenoids give the characteristic color to carrots, corn, canaries, and daffodils, as well as egg yolks, rutabagas, buttercups, and bananas. Carotenoids can be produced from fats and other basic organic metabolic building blocks by all these organisms. The only animals known to produce carotenoids are aphids and spider mites, which acquired the ability and genes from fungi or it is produced by endosymbiotic bacteria in whiteflies. Carotenoids from the diet are stored in the fatty tissues of animals, and exclusively carnivorous animals obtain the compounds from animal fat.
The quinones are a class of organic compounds that are formally "derived from aromatic compounds [such as benzene or naphthalene] by conversion of an even number of –CH= groups into –C(=O)– groups with any necessary rearrangement of double bonds", resulting in "a fully conjugated cyclic dione structure". The class includes some heterocyclic compounds.
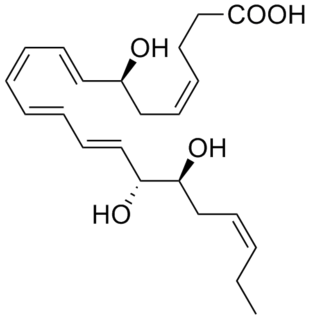
Resolvins are metabolic byproducts of omega-3 fatty acids, primarily eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), as well as docosapentaenoic acid (DPA) and clupanodonic acid. As autacoids similar to hormones acting on local tissues, resolvins are under preliminary research for their involvement in promoting restoration of normal cellular function following the inflammation that occurs after tissue injury. Resolvins belong to a class of polyunsaturated fatty acid (PUFA) metabolites termed specialized proresolving mediators (SPMs).

1,4-Benzoquinone, commonly known as para-quinone, is a chemical compound with the formula C6H4O2. In a pure state, it forms bright-yellow crystals with a characteristic irritating odor, resembling that of chlorine, bleach, and hot plastic or formaldehyde. This six-membered ring compound is the oxidized derivative of 1,4-hydroquinone. The molecule is multifunctional: it exhibits properties of a ketone, forming an oxime; an oxidant, forming the dihydroxy derivative; and an alkene, undergoing addition reactions, especially those typical for α,β-unsaturated ketones. 1,4-Benzoquinone is sensitive toward both strong mineral acids and alkali, which cause condensation and decomposition of the compound.
1,2-Benzoquinone, also called ortho-benzoquinone, is an organic compound with formula C6H4O2. It is one of the two isomers of quinone, the other being 1,4-benzoquinone. It is a red volatile solid that is soluble in water and ethyl ether. It is rarely encountered because of its instability, but it is of fundamental interest as the parent ortho-quinone, of which many analogues are known.
In enzymology, a 2-dehydro-3-deoxy-D-gluconate 6-dehydrogenase (EC 1.1.1.126) is an enzyme that catalyzes the chemical reaction
In enzymology, a stizolobinate synthase (EC 1.13.11.30) is an enzyme that catalyzes the chemical reaction
The molecular formula C8H8O4 (molar mass : 168.15 g/mol, exact mass : 168.042259 u) may refer to:

Tetrahydroxy-1,4-benzoquinone, also called tetrahydroxy-p-benzoquinone, tetrahydroxybenzoquinone, or tetrahydroxyquinone (THBQ, THQ), is an organic compound with formula C6O2(OH)4. Its molecular structure consists of a cyclohexadiene ring with four hydroxyl groups and two ketone groups in opposite (para) positions.

Chloranil is a quinone with the molecular formula C6Cl4O2. Also known as tetrachloro-1,4-benzoquinone, it is a yellow solid. Like the parent benzoquinone, chloranil is a planar molecule that functions as a mild oxidant.

Chloranilic acid is an organic compound with the chemical formula C6H2Cl2O4.
A hydroxybenzoquinone is any of several organic compounds that can be viewed as derivatives of a benzoquinone through replacement of one hydrogen atom (H) by a hydroxyl group (-OH).

In chemistry, 1,4-benzoquinonetetracarboxylic acid is an organic compound with formula C
10H
4O
10, or (C6O2)(-(CO)OH)4, which can be viewed as deriving from para-benzoquinone C
6H
4O
2 through replacement of the four hydrogen atoms by carboxyl functional groups -(CO)OH.

2,6-Dimethoxybenzoquinone (2,6-DMBQ) is a benzoquinone, a chemical compound found in Rauvolfia vomitoria and in Tibouchina pulchra.
Olivetolic acid cyclase is an enzyme with systematic name 3,5,7-trioxododecanoyl-CoA CoA-lyase (2,4-dihydroxy-6-pentylbenzoate-forming). This enzyme catalyses the following chemical reaction
The molecular formula C6H4O5 may refer to:

Polyporic acid is a para-terphenyl benzoquinone compound first identified by German chemist Stahlschmidt from a mycelial culture of the fungus species Hapalopilus nidulans in 1877. This chemical, present at 20–40% of the fresh weight of the fruit bodies, inhibits the enzyme dihydroorotate dehydrogenase. It is found in other mushrooms, but in much lower amounts.
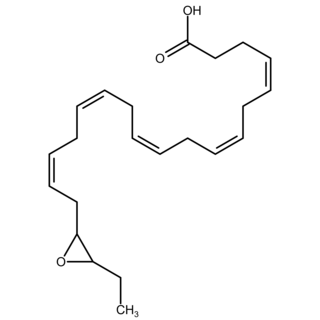
Epoxide docosapentaenoic acids are metabolites of the 22-carbon straight-chain omega-3 fatty acid, docosahexaenoic acid (DHA). Cell types that express certain cytochrome P450 (CYP) epoxygenases metabolize polyunsaturated fatty acid's (PUFAs) by converting one of their double bonds to an epoxide. In the best known of these metabolic pathways, cellular CYP epoxygenases metabolize the 20-carbon straight-chain omega-6 fatty acid, arachidonic acid, to epoxyeicosatrienoic acids (EETs); another CYP epoxygenase pathway metabolizes the 20-carbon omega-3 fatty acid, eicosapentaenoic acid (EPA), to epoxyeicosatetraenoic acids (EEQs). CYP epoxygenases similarly convert various other PUFAs to epoxides These epoxide metabolites have a variety of activities. However, essentially all of them are rapidly converted to their corresponding, but in general far less active, Vicinal (chemistry) dihydroxy fatty acids by ubiquitous cellular Soluble epoxide hydrolase. Consequently, these epoxides, including EDPs, operate as short-lived signaling agents that regulate the function of their parent or nearby cells. The particular feature of EDPs distinguishing them from EETs is that they derive from omega-3 fatty acids and are suggested to be responsible for some of the beneficial effects attributed to omega-3 fatty acids and omega-3-rich foods such as fish oil.











