Related Research Articles

Hepatotoxicity implies chemical-driven liver damage. Drug-induced liver injury (DILI) is a cause of acute and chronic liver disease caused specifically by medications and the most common reason for a drug to be withdrawn from the market after approval.
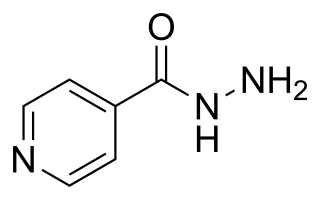
Isoniazid, also known as isonicotinic acid hydrazide (INH), is an antibiotic used for the treatment of tuberculosis. For active tuberculosis, it is often used together with rifampicin, pyrazinamide, and either streptomycin or ethambutol. For latent tuberculosis, it is often used alone. It may also be used for atypical types of mycobacteria, such as M. avium, M. kansasii, and M. xenopi. It is usually taken by mouth, but may be used by injection into muscle.
Toxication, toxification or toxicity exaltation is the conversion of a chemical compound into a more toxic form in living organisms or in substrates such as soil or water. The conversion can be caused by enzymatic metabolism in the organisms, as well as by abiotic chemical reactions. While the parent drug are usually less active, both the parent drug and its metabolite can be chemically active and cause toxicity, leading to mutagenesis, teratogenesis, and carcinogenesis. Different classes of enzymes, such as P450 monooxygenases, epoxide hydrolase, or acetyltransferases can catalyze the process in the cell, mostly in the liver.

Noni juice is derived from the fruit of the Morinda citrifolia tree indigenous to Southeast Asia and Australasia. It has been promoted, illegally in several cases, as a cure for a number of human diseases. However, there is no evidence to support any claims of therapeutic benefit.
Pharmacovigilance, also known as drug safety, is the pharmaceutical science relating to the "collection, detection, assessment, monitoring, and prevention" of adverse effects with pharmaceutical products. The etymological roots for the word "pharmacovigilance" are: pharmakon and vigilare. As such, pharmacovigilance heavily focuses on adverse drug reactions (ADR), which are defined as any response to a drug which is noxious and unintended, including lack of efficacy. Medication errors such as overdose, and misuse and abuse of a drug as well as drug exposure during pregnancy and breastfeeding, are also of interest, even without an adverse event, because they may result in an adverse drug reaction.
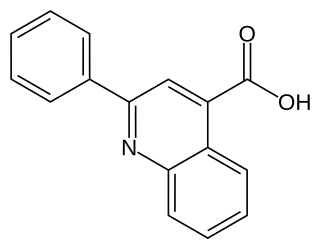
Cinchophen is an analgesic drug that was first produced by Doebner & Gieskel in 1887, it was commercially introduced in 1908 as a treatment for gout. This drug is still used, in combination with Prednisolone, by veterinarians to treat arthritis in animals. It can be prepared starting from anilin, benzaldehyde and pyruvic acid in absolute ethanol. Use of this drug in humans ceased in the 1930s due to the discovery that cinchophen can cause serious liver damage. There is some evidence that it stimulates C-Fos.

Pemoline, sold under the brand name Cylert among others, is a stimulant medication which has been used in the treatment of attention-deficit hyperactivity disorder (ADHD) and narcolepsy. It has been discontinued in most countries due to rare but serious problems with liver toxicity. The medication was taken by mouth.
Cholestasis is a condition where the flow of bile from the liver to the duodenum is impaired. The two basic distinctions are:

The Council for International Organizations of Medical Sciences (CIOMS) is an international non-governmental organization of 40 international, national, and associate member groups representing the biomedical science community. It was jointly established by the World Health Organization (WHO) and United Nations Educational, Scientific and Cultural Organization (UNESCO) in 1949 as a successor to the International Medical Congress that organized 17 conferences from 1867 until the 1913 outbreak of World War I.

Iproniazid is a non-selective, irreversible monoamine oxidase inhibitor (MAOI) of the hydrazine class. It is a xenobiotic that was originally designed to treat tuberculosis, but was later most prominently used as an antidepressant drug. However, it was withdrawn from the market because of its hepatotoxicity. The medical use of iproniazid was discontinued in most of the world in the 1960s, but remained in use in France until its discontinuation in 2015.

Flutamide, sold under the brand name Eulexin among others, is a nonsteroidal antiandrogen (NSAA) which is used primarily to treat prostate cancer. It is also used in the treatment of androgen-dependent conditions like acne, excessive hair growth, and high androgen levels in women. It is taken by mouth, usually three times per day.

NAPQI, also known as NAPBQI or N-acetyl-p-benzoquinone imine, is a toxic byproduct produced during the xenobiotic metabolism of the analgesic paracetamol (acetaminophen). It is normally produced only in small amounts, and then almost immediately detoxified in the liver.

Pyrrolizidine alkaloids (PAs), sometimes referred to as necine bases, are a group of naturally occurring alkaloids based on the structure of pyrrolizidine. Their use dates back centuries and is intertwined with the discovery, understanding, and eventual recognition of their toxicity on humans and animals.

Bentazepam is a thienodiazepine which is a benzodiazepine analog.
Benoxaprofen, also known as Benoxaphen, is a chemical compound with the formula C16H12ClNO3. It is a non-steroidal anti-inflammatory drug (NSAID) of the propionic acid class, and was marketed under the brand name Opren in the United Kingdom and Europe by Eli Lilly and Company (commonly referred to as Lilly), and as Oraflex in the United States of America (USA). Lilly suspended sales of Oraflex in 1982 after reports from the British government and the United States Food and Drug Administration (US FDA) of adverse effects and deaths linked to the drug.

Troxipide is a drug used in the treatment of gastroesophageal reflux disease. Troxipide is a systemic non-antisecretory gastric cytoprotective agent with anti-ulcer, anti-inflammatory and mucus secreting properties irrespective of pH of stomach or duodenum. Troxipide is currently marketed in Japan (Aplace), China (Shuqi), South Korea (Defensa), and India (Troxip). It is used for the management of gastric ulcers, and amelioration of gastric mucosal lesions in acute gastritis and acute exacerbation of chronic gastritis.
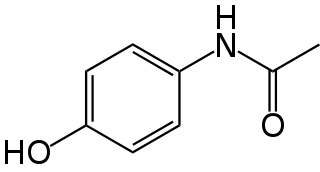
Paracetamol poisoning, also known as acetaminophen poisoning, is caused by excessive use of the medication paracetamol (acetaminophen). Most people have few or non-specific symptoms in the first 24 hours following overdose. These symptoms include feeling tired, abdominal pain, or nausea. This is typically followed by absence of symptoms for a couple of days, after which yellowish skin, blood clotting problems, and confusion occurs as a result of liver failure. Additional complications may include kidney failure, pancreatitis, low blood sugar, and lactic acidosis. If death does not occur, people tend to recover fully over a couple of weeks. Without treatment, death from toxicity occurs 4 to 18 days later.
Urelumab is a fully human, non‐ligand binding, CD137 agonist immunoglobulin‐γ 4 (IgG4) monoclonal antibody. It was developed utilizing Medarex's UltiMAb(R) technology by Bristol-Myers Squibb for the treatment of cancer and solid tumors. Urelumab promotes anti-tumor immunity, or an immune response against tumor cells, via CD137 activation. The application of Urelumab has been limited due to the fact that it can cause severe liver toxicity.

Chrysophanol, also known as chrysophanic acid, is a fungal isolate and a natural anthraquinone. It is a C-3 methyl substituted chrysazin of the trihydroxyanthraquinone family.
The side effects of bicalutamide, a nonsteroidal antiandrogen (NSAA), including its frequent and rare side effects, have been well-studied and characterized. The most common side effects of bicalutamide monotherapy in men include breast tenderness, breast growth, feminization, demasculinization, and hot flashes. Less common side effects of bicalutamide monotherapy in men include sexual dysfunction, depression, fatigue, weakness, and anemia. Bicalutamide is well tolerated and has few side effects in women. General side effects of bicalutamide that may occur in either sex include diarrhea, constipation, abdominal pain, nausea, dry skin, itching, and rash.
References
- 1 2 Andrade RJ, Robles M, Fernández-Castañer A, López-Ortega S, López-Vega MC, Lucena MI (2007). "Assessment of drug-induced hepatotoxicity in clinical practice: a challenge for gastroenterologists". World J. Gastroenterol. 13 (3): 329–40. doi: 10.3748/wjg.v13.i3.329 . PMC 4065885 . PMID 17230599.
- ↑ Arundel C, Lewis JH (2007). "Drug-induced liver disease in 2006". Curr. Opin. Gastroenterol. 23 (3): 244–54. doi:10.1097/MOG.0b013e3280b17dfb. PMID 17414839. S2CID 5842491.