Related Research Articles
In toxicology, the median lethal dose, LD50 (abbreviation for "lethal dose, 50%"), LC50 (lethal concentration, 50%) or LCt50 is a measure of the lethal dose of a toxin, radiation, or pathogen. The value of LD50 for a substance is the dose required to kill half the members of a tested population after a specified test duration. LD50 figures are frequently used as a general indicator of a substance's acute toxicity. A lower LD50 is indicative of increased toxicity.

A safety data sheet (SDS), material safety data sheet (MSDS), or product safety data sheet (PSDS) is a document that lists information relating to occupational safety and health for the use of various substances and products. SDSs are a widely used system for cataloguing information on chemicals, chemical compounds, and chemical mixtures. SDS information may include instructions for the safe use and potential hazards associated with a particular material or product, along with spill-handling procedures. The older MSDS formats could vary from source to source within a country depending on national requirements; however, the newer SDS format is internationally standardized.

Occupational hygiene is the anticipation, recognition, evaluation, control, and confirmation of protection from hazards at work that may result in injury, illness, or affect the well being of workers. These hazards or stressors are typically divided into the categories biological, chemical, physical, ergonomic and psychosocial. The risk of a health effect from a given stressor is a function of the hazard multiplied by the exposure to the individual or group. For chemicals, the hazard can be understood by the dose response profile most often based on toxicological studies or models. Occupational hygienists work closely with toxicologists for understanding chemical hazards, physicists for physical hazards, and physicians and microbiologists for biological hazards Environmental and occupational hygienists are considered experts in exposure science and exposure risk management. Depending on an individual's type of job, a hygienist will apply their exposure science expertise for the protection of workers, consumers and/or communities.
This page provides supplementary chemical data on acetic acid.
This page provides supplementary chemical data on acetone.
This page provides supplementary chemical data on formic acid.
This page provides supplementary chemical data on ammonia.
This page provides supplementary chemical data on boric acid.
This page provides supplementary chemical data on ethyl acetate.
This page provides supplementary chemical data on barium nitrate.
This page provides supplementary chemical data on calcium hydroxide.
This page provides supplementary chemical data on butanone.
This page provides supplementary chemical data on aniline.
This page provides supplementary chemical data on ethylene glycol.
This page provides supplementary chemical data on chloroform.
This page provides supplementary chemical data on cyclohexane.
This page provides supplementary chemical data on n-hexane.
This page provides supplementary chemical data on bromoform.
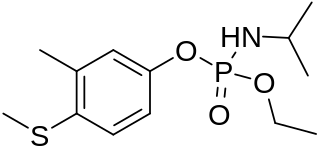
Fenamiphos is an organophosphate acetylcholinesterase inhibitor used as an insecticide.
Hazard substitution is a hazard control strategy in which a material or process is replaced with another that is less hazardous. Substitution is the second most effective of the five members of the hierarchy of hazard controls in protecting workers, after elimination. Substitution and elimination are most effective early in the design process, when they may be inexpensive and simple to implement, while for an existing process they may require major changes in equipment and procedures. The concept of prevention through design emphasizes integrating the more effective control methods such as elimination and substitution early in the design phase.
References
Except where noted otherwise, data relate to standard ambient temperature and pressure (STP).
Disclaimer applies.