Carbonate may refer to:
Carbonate may refer to:
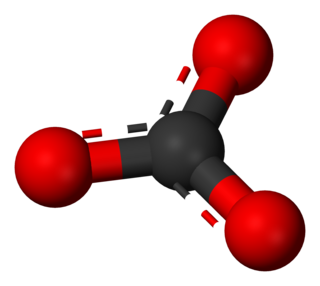
A carbonate is a salt of carbonic acid (H2CO3), characterized by the presence of the carbonate ion, a polyatomic ion with the formula CO2−
3. The word carbonate may also refer to a carbonate ester, an organic compound containing the carbonate group C(=O)(O–)2.
In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively charged sodium ions and negatively charged chloride ions.

In chemistry, a silicate is any member of a family of polyatomic anions consisting of silicon and oxygen, usually with the general formula [SiO(4-2x)−
4−x]
n, where 0 ≤ x < 2. The family includes orthosilicate SiO4−4, metasilicate SiO2−3, and pyrosilicate Si2O6−7. The name is also used for any salt of such anions, such as sodium metasilicate; or any ester containing the corresponding chemical group, such as tetramethyl orthosilicate.

Chalcopyrite ( KAL-kə-PY-ryte, -koh-) is a copper iron sulfide mineral and the most abundant copper ore mineral. It has the chemical formula CuFeS2 and crystallizes in the tetragonal system. It has a brassy to golden yellow color and a hardness of 3.5 to 4 on the Mohs scale. Its streak is diagnostic as green-tinged black.

Aragonite is a carbonate mineral, one of the three most common naturally occurring crystal forms of calcium carbonate, CaCO3. It is formed by biological and physical processes, including precipitation from marine and freshwater environments.

Azurite is a soft, deep-blue copper mineral produced by weathering of copper ore deposits. During the early 19th century, it was also known as chessylite, after the type locality at Chessy-les-Mines near Lyon, France. The mineral, a basic carbonate with the chemical formula Cu3(CO3)2(OH)2, has been known since ancient times, and was mentioned in Pliny the Elder's Natural History under the Greek name kuanos (κυανός: "deep blue," root of English cyan) and the Latin name caeruleum. Since antiquity, azurite's exceptionally deep and clear blue has been associated with low-humidity desert and winter skies. The modern English name of the mineral reflects this association, since both azurite and azure are derived via Arabic from the Persian lazhward (لاژورد), an area known for its deposits of another deep-blue stone, lapis lazuli ("stone of azure").

Chromate salts contain the chromate anion, CrO2−
4. Dichromate salts contain the dichromate anion, Cr
2O2−
7. They are oxyanions of chromium in the +6 oxidation state and are moderately strong oxidizing agents. In an aqueous solution, chromate and dichromate ions can be interconvertible.

Calcium citrate is the calcium salt of citric acid. It is commonly used as a food additive (E333), usually as a preservative, but sometimes for flavor. In this sense, it is similar to sodium citrate. Calcium citrate is also found in some dietary calcium supplements. Calcium makes up 24.1% of calcium citrate (anhydrous) and 21.1% of calcium citrate (tetrahydrate) by mass. The tetrahydrate occurs in nature as the mineral Earlandite.

Alkalinity is the capacity of water to resist acidification. It should not be confused with basicity, which is an absolute measurement on the pH scale.

Carbonate minerals are those minerals containing the carbonate ion, CO2−
3.

Hexafluorosilicic acid is an inorganic compound with the chemical formula H
2SiF
6. Aqueous solutions of hexafluorosilicic acid consist of salts of the cation and hexafluorosilicate anion. These salts and their aqueous solutions are colorless.

Ettringite is a hydrous calcium aluminium sulfate mineral with formula: Ca6Al2(SO4)3(OH)12·26H2O. It is a colorless to yellow mineral crystallizing in the trigonal system. The prismatic crystals are typically colorless, turning white on partial dehydration. It is part of the ettringite-group which includes other sulfates such as thaumasite and bentorite.

Green rust is a generic name for various green crystalline chemical compounds containing iron(II) and iron(III) cations, the hydroxide (HO−
) anion, and another anion such as carbonate (CO2−
3), chloride (Cl−
), or sulfate (SO2−
4), in a layered double hydroxide structure. The most studied varieties are
The purpose of a mineralizer is to facilitate the transport of insoluble “nutrient” to a seed crystal by means of a reversible chemical reaction. Over time, the seed crystal accumulates the material that was once in the nutrient and grows. Mineralizers are additives that aid the solubilization of the nutrient solid. When used in small quantities, mineralizers function as catalysts. Typically, a more stable solid is crystallized from a solution that consists of a less stable solid and a solvent. The process is done by dissolution-precipitation or crystallization process.

Donnayite-(Y) is a rare-earth carbonate mineral containing the rare-earth metal yttrium. It was first discovered in 1978 at Mont Saint-Hilaire, Quebec. Donnayite was subsequently identified and named after Joseph D. H. Donnay and his wife, Gabrielle Donnay. Both were prominent mineralogists and crystallographers, and J. D. H. Donnay was awarded the Roebling Award by the Mineralogical Society of America in 1971 for his emphasis on the importance of optical mineralogy and crystal morphology. Donnayite tends to occur in small quantities in the pegmatite dykes and miarolitic cavities of mountainous regions. It crystallizes in this environment with increasing alkalinity values until the alkalinity suddenly drops during the last stage of crystallization. This results in increasing amounts of Na carbonates and REE minerals. First discovered at Mont St-Hilaire, donnayite has since been found in the Southern Ural Mountains of Russia and the Narssarssuk pegmatite of South Greenland. Donnayite crystals tend to be small and the color is commonly pale yellow to yellow with a white streak and a vitreous luster. Donnayite crystals usually display trigonal or hexagonal symmetry and have a hardness of 3. Twinning is extremely common in this mineral. Minerals closely related to donnayite include synchysite, calcite, sphalerite, microcline, and analcime. Donnayite is isomorphous with weloganite and mckelveyite.

Rapidcreekite is a rare mineral with formula Ca2(SO4)(CO3)·4H2O. The mineral is white to colorless and occurs as groupings of acicular (needle-shaped) crystals. It was discovered in 1983 in northern Yukon, Canada, and described in 1986. Rapidcreekite is structurally and compositionally similar to gypsum.

Copper(II) carbonate or cupric carbonate is a chemical compound with formula CuCO
3. At ambient temperatures, it is an ionic solid consisting of copper(II) cations Cu2+
and carbonate anions CO2−
3.
Bijvoetite-(Y) is a very rare rare-earth and uranium mineral with the formula (Y,REE)8(UO2)16(CO3)16O8(OH)8·39H2O. When compared to the original description, the formula of bijvoetite-(Y) was changed in the course of crystal structure redefinition. Bijvoetite-(Y) is an example of natural salts containing both uranium and yttrium, the other examples being kamotoite-(Y) and sejkoraite-(Y). Bijvoetite-(Y) comes from Shinkolobwe deposit in Republic of Congo, which is famous for rare uranium minerals. The other interesting rare-earth-bearing uranium mineral, associated with bijvoetite-(Y), is lepersonnite-(Gd).
The carbonate chlorides are double salts containing both carbonate and chloride anions. Quite a few minerals are known. Several artificial compounds have been made. Some complexes have both carbonate and chloride ligands. They are part of the family of halocarbonates. In turn these halocarbonates are a part of mixed anion materials.
The borate carbonates are mixed anion compounds containing both borate and carbonate ions. Compared to mixed anion compounds containing halides, these are quite rare. They are hard to make, requiring higher temperatures, which are likely to decompose carbonate to carbon dioxide. The reason for the difficulty of formation is that when entering a crystal lattice, the anions have to be correctly located, and correctly oriented. They are also known as borocarbonates. Although these compounds have been termed carboborate, that word also refers to the C=B=C5− anion, or CB11H12− anion. This last anion should be called 1-carba-closo-dodecaborate or monocarba-closo-dodecaborate.