Cerium fluoride may refer to:
- Cerium(III) fluoride (neodymium trifluoride), CeF3
- Cerium(IV) fluoride (neodymium tetrafluoride), CeF4
Cerium fluoride may refer to:

Lanthanum is a chemical element with the symbol La and atomic number 57. It is a soft, ductile, silvery-white metal that tarnishes slowly when exposed to air. It is the eponym of the lanthanide series, a group of 15 similar elements between lanthanum and lutetium in the periodic table, of which lanthanum is the first and the prototype. Lanthanum is traditionally counted among the rare earth elements. Like most other rare earth elements, the usual oxidation state is +3. Lanthanum has no biological role in humans but is essential to some bacteria. It is not particularly toxic to humans but does show some antimicrobial activity.

Neodymium is a chemical element with the symbol Nd and atomic number 60. It is the fourth member of the lanthanide series and is considered to be one of the rare-earth metals. It is a hard, slightly malleable, silvery metal that quickly tarnishes in air and moisture. When oxidized, neodymium reacts quickly producing pink, purple/blue and yellow compounds in the +2, +3 and +4 oxidation states. It is generally regarded as having one of the most complex spectra of the elements. Neodymium was discovered in 1885 by the Austrian chemist Carl Auer von Welsbach, who also discovered praseodymium. It is present in significant quantities in the minerals monazite and bastnäsite. Neodymium is not found naturally in metallic form or unmixed with other lanthanides, and it is usually refined for general use. Neodymium is fairly common—about as common as cobalt, nickel, or copper—and is widely distributed in the Earth's crust. Most of the world's commercial neodymium is mined in China, as is the case with many other rare-earth metals.

Gadolinite, sometimes known as ytterbite, is a silicate mineral consisting principally of the silicates of cerium, lanthanum, neodymium, yttrium, beryllium, and iron with the formula (Ce,La,Nd,Y)2FeBe2Si2O10. It is called gadolinite-(Ce) or gadolinite-(Y), depending on the prominent composing element. It may contain 35.5% yttria sub-group rare earths, 2.2% ceria earths, as much as to 11.6% BeO, and traces of thorium. It is found in Sweden, Norway, and the US.

The mineral bastnäsite (or bastnaesite) is one of a family of three carbonate-fluoride minerals, which includes bastnäsite-(Ce) with a formula of (Ce, La)CO3F, bastnäsite-(La) with a formula of (La, Ce)CO3F, and bastnäsite-(Y) with a formula of (Y, Ce)CO3F. Some of the bastnäsites contain OH− instead of F− and receive the name of hydroxylbastnasite. Most bastnäsite is bastnäsite-(Ce), and cerium is by far the most common of the rare earths in this class of minerals. Bastnäsite and the phosphate mineral monazite are the two largest sources of cerium and other rare-earth elements.

Praseodymium is a chemical element with the symbol Pr and the atomic number 59. It is the third member of the lanthanide series and is considered to be one of the rare-earth metals. It is a soft, silvery, malleable and ductile metal, valued for its magnetic, electrical, chemical, and optical properties. It is too reactive to be found in native form, and pure praseodymium metal slowly develops a green oxide coating when exposed to air.

Cerium(III) chloride (CeCl3), also known as cerous chloride or cerium trichloride, is a compound of cerium and chlorine. It is a white hygroscopic salt; it rapidly absorbs water on exposure to moist air to form a hydrate, which appears to be of variable composition, though the heptahydrate CeCl3·7H2O is known. It is highly soluble in water, and (when anhydrous) it is soluble in ethanol and acetone.
Neodymium(III) chloride or neodymium trichloride is a chemical compound of neodymium and chlorine with the formula NdCl3. This anhydrous compound is a mauve-colored solid that rapidly absorbs water on exposure to air to form a purple-colored hexahydrate, NdCl3·6H2O. Neodymium(III) chloride is produced from minerals monazite and bastnäsite using a complex multistage extraction process. The chloride has several important applications as an intermediate chemical for production of neodymium metal and neodymium-based lasers and optical fibers. Other applications include a catalyst in organic synthesis and in decomposition of waste water contamination, corrosion protection of aluminium and its alloys, and fluorescent labeling of organic molecules (DNA).

Parisite is a rare mineral consisting of cerium, lanthanum and calcium fluoro-carbonate, Ca(Ce,La)2(CO3)3F2. Parisite is mostly parisite-(Ce), but when neodymium is present in the structure the mineral becomes parisite-(Nd).
CEF4 or CeF4 could signify:
CEF3 or CeF3 could signify:
Uranyl carbonate refers to the inorganic compound with the formula UO2CO3. Also known by its mineral name rutherfordine, this material consists of uranyl (UO22+) and carbonate (CO32-). Like most uranyl salts, the compound is a polymeric, each uranium(VI) center being bonded to eight O atoms. Hydrolysis products of rutherfordine are also found in both the mineral and organic fractions of coal and its fly ash and is the main component of uranium in mine tailing seepage water.

Cerium is a chemical element with the symbol Ce and atomic number 58. Cerium is a soft, ductile, and silvery-white metal that tarnishes when exposed to air. Cerium is the second element in the lanthanide series, and while it often shows the +3 oxidation state characteristic of the series, it also has a stable +4 state that does not oxidize water. It is also considered one of the rare-earth elements. Cerium has no known biological role in humans but is not particularly toxic, except with intense or continued exposure.

Cerium(III) fluoride (or cerium trifluoride), CeF3, is an ionic compound of the rare earth metal cerium and fluorine.

Neodymium(III) fluoride is an inorganic chemical compound of neodymium and fluorine with the formula NdF3. It is a purplish pink colored solid with a high melting point.

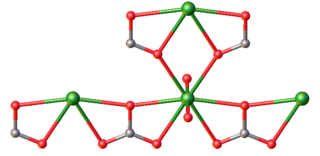
A carbonate fluoride, fluoride carbonate, fluorocarbonate or fluocarbonate is a double salt containing both carbonate and fluoride. The salts are usually insoluble in water, and can have more than one kind of metal cation to make more complex compounds. Rare-earth fluorocarbonates are particularly important as ore minerals for the light rare-earth elements lanthanum, cerium and neodymium. Bastnäsite is the most important source of these elements. Other artificial compounds are under investigation as non-linear optical materials and for transparency in the ultraviolet, with effects over a dozen times greater than Potassium dideuterium phosphate.
Praseodymium(III) fluoride is an inorganic compound with the formula PrF3, being the most stable fluoride of praseodymium.
Cerium(IV) fluoride is an inorganic compound with a chemical formula CeF4. It is a strong oxidant that appears as a white crystalline material. Cerium(IV) fluoride has an anhydrous form and a monohydrate form.
Cerium(III) sulfide, also known as cerium sesquisulfide, is an inorganic compound with the formula Ce2S3. It is the sulfide salt of cerium(III) and exists as three polymorphs with different crystal structures.
Praseodymium compounds are compounds formed by the lanthanide metal praseodymium (Pr). In these compounds, praseodymium generally exhibits the +3 oxidation state, such as PrCl3, Pr(NO3)3 and Pr(CH3COO)3. However, compounds with praseodymium in the +2 and +4 oxidation states, and unlike other lanthanides, the +5 oxidation state, are also known.