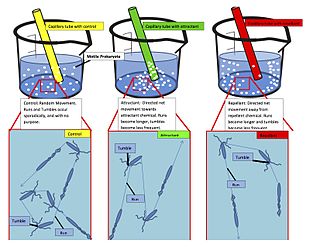
Chemotaxis is the movement of an organism or entity in response to a chemical stimulus. Somatic cells, bacteria, and other single-cell or multicellular organisms direct their movements according to certain chemicals in their environment. This is important for bacteria to find food by swimming toward the highest concentration of food molecules, or to flee from poisons. In multicellular organisms, chemotaxis is critical to early development and development as well as in normal function and health. In addition, it has been recognized that mechanisms that allow chemotaxis in animals can be subverted during cancer metastasis. The aberrant chemotaxis of leukocytes and lymphocytes also contribute to inflammatory diseases such as atherosclerosis, asthma, and arthritis. Sub-cellular components, such as the polarity patch generated by mating yeast, may also display chemotactic behavior.

Cytokines are a broad and loose category of small proteins important in cell signaling. Cytokines are peptides and cannot cross the lipid bilayer of cells to enter the cytoplasm. Cytokines have been shown to be involved in autocrine, paracrine and endocrine signaling as immunomodulating agents. Their definite distinction from hormones is still part of ongoing research.

Chemokines, or chemotactic cytokines, are a family of small cytokines or signaling proteins secreted by cells that induce directional movement of leukocytes, as well as other cell types, including endothelial and epithelial cells. In addition to playing a major role in the activation of host immune responses, chemokines are important for biological processes, including morphogenesis and wound healing, as well as in the pathogenesis of diseases like cancers.

Interleukin 8 is a chemokine produced by macrophages and other cell types such as epithelial cells, airway smooth muscle cells and endothelial cells. Endothelial cells store IL-8 in their storage vesicles, the Weibel-Palade bodies. In humans, the interleukin-8 protein is encoded by the CXCL8 gene. IL-8 is initially produced as a precursor peptide of 99 amino acids which then undergoes cleavage to create several active IL-8 isoforms. In culture, a 72 amino acid peptide is the major form secreted by macrophages.

Chemokine ligand 5 is a protein which in humans is encoded by the CCL5 gene. The gene has been discovered in 1990 by in situ hybridisation and it is localised on 17q11.2-q12 chromosome. It is also known as RANTES. RANTES was first described by Dr. Tom Schall who named the protein, the original source of the name Rantes was from the Argentine movie Man Facing Southeast about an alien who shows up in a mental ward who was named Rantés, the rather clunky acronym was only made to fit the name.

Macrophage Inflammatory Proteins (MIP) belong to the family of chemotactic cytokines known as chemokines. In humans, there are two major forms, MIP-1α and MIP-1β that are now officially named CCL3 and CCL4, respectively. But we can sometimes encounter other names, especially in older literature, as LD78α, AT 464.1 and GOS19-1 for human CCL3 and AT 744, Act-2, LAG-1, HC21 and G-26 for human CCL4. Other macrophage inflammatory proteins include MIP-2, MIP-3 and MIP-5.

Interleukin 17 family is a family of pro-inflammatory cystine knot cytokines. They are produced by a group of T helper cell known as T helper 17 cell in response to their stimulation with IL-23. Originally, Th17 was identified in 1993 by Rouvier et al. who isolated IL17A transcript from a rodent T-cell hybridoma. The protein encoded by IL17A is a founding member of IL-17 family. IL17A protein exhibits a high homology with a viral IL-17-like protein encoded in the genome of T-lymphotropic rhadinovirus Herpesvirus saimiri. In rodents, IL-17A is often referred to as CTLA8.

Chemokine ligand 7 (CCL7) is a small cytokine that was previously called monocyte-chemotactic protein 3 (MCP3). CCL7 is a small protein that belongs to the CC chemokine family and is most closely related to CCL2.

Chemokine ligand 18 (CCL18) is a small cytokine belonging to the CC chemokine family. The functions of CCL18 have been well studied in laboratory settings, however the physiological effects of the molecule in living organisms have been difficult to characterize because there is no similar protein in rodents that can be studied. The receptor for CCL18 has been identified in humans only recently, which will help scientists understand the molecule's role in the body.
Chemokine ligand 16 (CCL16) is a small cytokine belonging to the CC chemokine family that is known under several pseudonyms, including Liver-expressed chemokine (LEC) and Monotactin-1 (MTN-1). This chemokine is expressed by the liver, thymus, and spleen and is chemoattractive for monocytes and lymphocytes. Cellular expression of CCL16 can be strongly induced in monocytes by IL-10, IFN-γ and bacterial lipopolysaccharide. Its gene is located on chromosome 17, in humans, among a cluster of other CC chemokines. CCL16 elicits its effects on cells by interacting with cell surface chemokine receptors such as CCR1, CCR2, CCR5 and CCR8.
Chemokine ligand 24 (CCL24) also known as myeloid progenitor inhibitory factor 2 (MPIF-2) or eosinophil chemotactic protein 2 (eotaxin-2) is a protein that in humans is encoded by the CCL24 gene. This gene is located on human chromosome 7.

C-X-C motif chemokine 11 (CXCL11) is a protein that in humans is encoded by the CXCL11 gene.
The formyl peptide receptors (FPR) belong to a class of G protein-coupled receptors involved in chemotaxis. In humans, there are three formyl peptide receptor isoforms, each encoded by a separate gene that are named FPR1, FPR2, and FPR3. These receptors were originally identified by their ability to bind N-formyl peptides such as N-formylmethionine produced by the degradation of either bacterial or host cells. Hence formyl peptide receptors are involved in mediating immune cell response to infection. These receptors may also act to suppress the immune system under certain conditions. The close phylogenetic relation of signaling in chemotaxis and olfaction was recently proved by detection formyl peptide receptor like proteins as a distinct family of vomeronasal organ chemosensors in mice.

Leukocyte extravasation is the movement of leukocytes out of the circulatory system and towards the site of tissue damage or infection. This process forms part of the innate immune response, involving the recruitment of non-specific leukocytes. Monocytes also use this process in the absence of infection or tissue damage during their development into macrophages.

N-Formylmethionyl-leucyl-phenylalanine is an N-formylated tripeptide and sometimes simply referred to as chemotactic peptide is a potent polymorphonuclear leukocyte (PMN) chemotactic factor and is also a macrophage activator.

Prostaglandin D2 receptor 2 (DP2 or CRTH2) is a human protein encoded by the PTGDR2 gene and GPR44. DP2 has also been designated as CD294 (cluster of differentiation 294). It is a member of the class of prostaglandin receptors which bind with and respond to various prostaglandins. DP2 along with Prostaglandin DP1 receptor are receptors for prostaglandin D2 (PGD2). Activation of DP2 by PGD2 or other cognate receptor ligands has been associated with certain physiological and pathological responses, particularly those associated with allergy and inflammation, in animal models and certain human diseases.
Interleukin-28 receptor is a type II cytokine receptor found largely in epithelial cells. It binds type 3 interferons, interleukin-28 A, Interleukin-28B, interleukin 29 and interferon lambda 4. It consists of an α chain and shares a common β subunit with the interleukin-10 receptor. Binding to the interleukin-28 receptor, which is restricted to select cell types, is important for fighting infection. Binding of the type 3 interferons to the receptor results in activation of the JAK/STAT signaling pathway.
Eugene C. "Gene" Butcher is an American immunologist and a professor of pathology at Stanford University.
Chemorepulsion is the directional movement of a cell away from a substance. Of the two directional varieties of chemotaxis, chemoattraction has been studied to a much greater extent. Only recently have the key components of the chemorepulsive pathway been elucidated. The exact mechanism is still being investigated, and its constituents are currently being explored as likely candidates for immunotherapies.












