
Chromium is a chemical element with the symbol Cr and atomic number 24. It is the first element in group 6. It is a steely-grey, lustrous, hard and brittle transition metal. Chromium is the main additive in stainless steel, to which it adds anti-corrosive properties. Chromium is also highly valued as a metal that is able to be highly polished while resisting tarnishing. Polished chromium reflects almost 70% of the visible spectrum, with almost 90% of infrared light being reflected. The name of the element is derived from the Greek word χρῶμα, chrōma, meaning color, because many chromium compounds are intensely colored.

Stainless steel is a group of iron-based alloys that contain a minimum of approximately 11% chromium, a composition that prevents the iron from rusting and also provides heat-resistant properties. Different types of stainless steel include the elements carbon, nitrogen, aluminium, silicon, sulfur, titanium, nickel, copper, selenium, niobium, and molybdenum. Specific types of stainless steel are often designated by a three-digit number, e.g., 304 stainless.

Group 6, numbered by IUPAC style, is a group of elements in the periodic table. Its members are chromium (Cr), molybdenum (Mo), tungsten (W), and seaborgium (Sg). These are all transition metals and chromium, molybdenum and tungsten are refractory metals. The period 8 elements of group 6 are likely to be either unpenthexium (Uph) or unpentoctium (Upo). This may not be possible; drip instability may imply that the periodic table ends around unbihexium. Neither unpenthexium nor unpentoctium have been synthesized, and it is unlikely that this will happen in the near future.

The term chromic acid is usually used for a mixture made by adding concentrated sulfuric acid to a dichromate, which may contain a variety of compounds, including solid chromium trioxide. This kind of chromic acid may be used as a cleaning mixture for glass. Chromic acid may also refer to the molecular species, H2CrO4 of which the trioxide is the anhydride. Chromic acid features chromium in an oxidation state of +6 (or VI). It is a strong and corrosive oxidising agent.

Chrome plating, often referred to simply as chrome, is a technique of electroplating a thin layer of chromium onto a metal object. The chromed layer can be decorative, provide corrosion resistance, ease cleaning procedures, or increase surface hardness. Sometimes, a less expensive imitator of chrome may be used for aesthetic purposes.

Chromium(III) chloride (also called chromic chloride) describes any of several compounds of with the formula CrCl3 • xH2O, where x can be 0, 5, and 6. The anhydrous compound with the formula CrCl3 is a violet solid. The most common form of the trichloride is the dark green hexahydrate, CrCl3 • 6H2O. Chromium chlorides find use as catalysts and as precursors to dyes for wool.

Chromium(III) oxide is an inorganic compound with the formula Cr
2O
3. It is one of the principal oxides of chromium and is used as a pigment. In nature, it occurs as the rare mineral eskolaite.

Chromium(III) fluoride is the name for the inorganic compounds with the chemical formula CrF3 as well as several related hydrates. The compound CrF3 is a green crystalline solid that is insoluble in common solvents, but the coloured hydrates [Cr(H2O)6]F3 and [Cr(H2O)6]F3•3H2O are soluble in water. The trihydrate is green, and the hexahydrate is violet. The anhydrous form sublimes at 1100–1200 °C.

Potassium chromate is the inorganic compound with the formula (K2CrO4). This yellow solid is the potassium salt of the chromate anion. It is a common laboratory chemical, whereas sodium chromate is important industrially.

Chromium(II) chloride describes inorganic compounds with the formula CrCl2(H2O)n. The anhydrous solid is white when pure, however commercial samples are often grey or green; it is hygroscopic and readily dissolves in water to give bright blue air-sensitive solutions of the tetrahydrate Cr(H2O)4Cl2. Chromium(II) chloride has no commercial uses but is used on a laboratory-scale for the synthesis of other chromium complexes.

Chromyl chloride is the inorganic compound with the formula CrO2Cl2. It is a reddish brown compound that is a volatile liquid at room temperature, which is unusual for transition metal complexes.
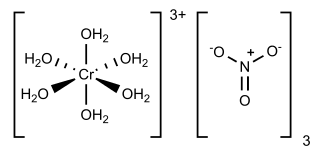
Chromium(III) nitrate describes several inorganic compounds consisting of chromium, nitrate and varying amounts of water. Most common is the dark violet hydrated solid, but an anhydrous green form is also known. Chromium(III) nitrate compounds are of a limited importance commercially, finding some applications in the dyeing industry. It is common in academic laboratories for the synthesis of chromium coordination complexes.
Chromium(IV) chloride (CrCl4) is an unstable chromium compound. It is generated by combining chromium(III) chloride and chlorine gas at elevated temperatures, but reverts to those substances at room temperature.

Takai olefination in organic chemistry describes the organic reaction of an aldehyde with a diorganochromium compound to form an alkene. It is a name reaction, referencing Kazuhiko Takai, who first reported it in 1986. In the original reaction, the organochromium species is generated from iodoform or bromoform and an excess of chromium(II) chloride and the product is a vinyl halide. One main advantage of this reaction is the E-configuration of the double bond that is formed. According to the original report, existing alternatives such as the Wittig reaction only gave mixtures.

The Nozaki–Hiyama–Kishi reaction is a nickel/chromium coupling reaction forming an alcohol from the reaction of an aldehyde with an allyl or vinyl halide. In their original 1977 publication, Tamejiro Hiyama and Hitoshi Nozaki reported on a chromium(II) salt solution prepared by reduction of chromic chloride by lithium aluminium hydride to which was added benzaldehyde and allyl chloride:
Chromium(II) fluoride is an inorganic compound with the formula CrF2. It exists as a blue-green iridescent solid. Chromium(II) fluoride is sparingly soluble in water, almost insoluble in alcohol, and is soluble in boiling hydrochloric acid, but is not attacked by hot distilled sulfuric acid or nitric acid. Like other chromous compounds, chromium(II) fluoride is oxidized to chromium(III) oxide in air.
Chromium pentafluoride is the inorganic compound with the chemical formula CrF5. It is a red volatile solid that melts at 34 °C. It is the highest known chromium fluoride, since the hypothetical chromium hexafluoride has not yet been synthesized.
Chromium(III) iodide, also known as chromium triiodide, is an inorganic compound with the formula CrI3. It is a black solid that is used to prepare other chromium compounds.

SAE 304 stainless steel is the most common stainless steel. The steel contains both chromium and nickel [1] metals as the main non-iron constituents. It is an austenitic stainless steel. It is less electrically and thermally conductive than carbon steel and is essentially-magnetic but less magnetic than steel. It has a higher corrosion resistance than regular steel and is widely used because of the ease in which it is formed into various shapes.

Chromium(III) acetate, commonly known as basic chromium acetate, describes a family of salts where the cation has the formula [Cr3O(O2CCH3)6(OH2)3]+. The trichromium cation is encountered with a variety of anions, such as chloride and nitrate. Data in the table above are for the chloride hexahydrate, [Cr3O(O2CCH3)6(OH2)3]Cl(H2O)6.















