
Keratin is one of a family of structural fibrous proteins also known as scleroproteins. Alpha-keratin (α-keratin) is a type of keratin found in vertebrates. It is the key structural material making up scales, hair, nails, feathers, horns, claws, hooves, and the outer layer of skin among vertebrates. Keratin also protects epithelial cells from damage or stress. Keratin is extremely insoluble in water and organic solvents. Keratin monomers assemble into bundles to form intermediate filaments, which are tough and form strong unmineralized epidermal appendages found in reptiles, birds, amphibians, and mammals. Excessive keratinization participate in fortification of certain tissues such as in horns of cattle and rhinos, and armadillos' osteoderm. The only other biological matter known to approximate the toughness of keratinized tissue is chitin. Keratin comes in two types, the primitive, softer forms found in all vertebrates and harder, derived forms found only among sauropsids.

An incandescent light bulb, incandescent lamp or incandescent light globe is an electric light with a wire filament heated until it glows. The filament is enclosed in a glass bulb with a vacuum or inert gas to protect the filament from oxidation. Current is supplied to the filament by terminals or wires embedded in the glass. A bulb socket provides mechanical support and electrical connections.
The word filament, which is descended from Latin filum meaning "thread", is used in English for a variety of thread-like structures, including:
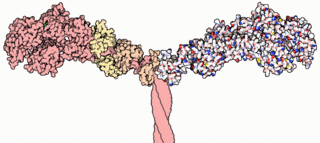
Myosins are a superfamily of motor proteins best known for their roles in muscle contraction and in a wide range of other motility processes in eukaryotes. They are ATP-dependent and responsible for actin-based motility. The term was originally used to describe a group of similar ATPases found in the cells of both striated muscle tissue and smooth muscle tissue. Following the discovery by Pollard and Korn (1973) of enzymes with myosin-like function in Acanthamoeba castellanii, a global range of divergent myosin genes have been discovered throughout the realm of eukaryotes.

Intermediate filaments (IFs) are cytoskeletal structural components found in the cells of vertebrates, and many invertebrates. Homologues of the IF protein have been noted in an invertebrate, the cephalochordate Branchiostoma.

A plasma globe or plasma lamp is a clear glass container/ball filled with a mixture of various noble gases with a high-voltage electrode in the center of the container.
Internexin, alpha-internexin, is a Class IV intermediate filament approximately 66 KDa. The protein was originally purified from rat optic nerve and spinal cord. The protein copurifies with other neurofilament subunits, as it was originally discovered, however in some mature neurons it can be the only neurofilament expressed. The protein is present in developing neuroblasts and in the Central Nervous System of adults. The protein is a major component of the intermediate filament network in small interneurons and cerebellar granule cells, where it is present in the parallel fibers.

Desmin is a protein that in humans is encoded by the DES gene. Desmin is a muscle-specific, type III intermediate filament that integrates the sarcolemma, Z disk, and nuclear membrane in sarcomeres and regulates sarcomere architecture.

Vimentin is a structural protein that in humans is encoded by the VIM gene. Its name comes from the Latin vimentum which refers to an array of flexible rods.

Peripherin is a type III intermediate filament protein expressed mainly in neurons of the peripheral nervous system. It is also found in neurons of the central nervous system that have projections toward peripheral structures, such as spinal motor neurons. Its size, structure, and sequence/location of protein motifs is similar to other type III intermediate filament proteins such as desmin, vimentin and glial fibrillary acidic protein. Like these proteins, peripherin can self-assemble to form homopolymeric filamentous networks, but it can also heteropolymerize with neurofilaments in several neuronal types. This protein in humans is encoded by the PRPH gene. Peripherin is thought to play a role in neurite elongation during development and axonal regeneration after injury, but its exact function is unknown. It is also associated with some of the major neuropathologies that characterize amyotropic lateral sclerosis (ALS), but despite extensive research into how neurofilaments and peripherin contribute to ALS, their role in this disease is still unidentified.
Coil or COIL may refer to:
Crescentin is a protein which is a bacterial relative of the intermediate filaments found in eukaryotic cells. Just as tubulins and actins, the other major cytoskeletal proteins, have prokaryotic homologs in, respectively, the FtsZ and MreB proteins, intermediate filaments are linked to the crescentin protein. Some of its homologs are erroneously labelled Chromosome segregation protein ParA. This protein family is found in Caulobacter and Methylobacterium.

Keratin 5, also known as KRT5, K5, or CK5, is a protein that is encoded in humans by the KRT5 gene. It dimerizes with keratin 14 and forms the intermediate filaments (IF) that make up the cytoskeleton of basal epithelial cells. This protein is involved in several diseases including epidermolysis bullosa simplex and breast and lung cancers.
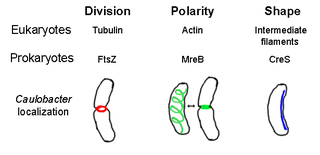
The prokaryotic cytoskeleton is the collective name for all structural filaments in prokaryotes. It was once thought that prokaryotic cells did not possess cytoskeletons, but advances in visualization technology and structure determination led to the discovery of filaments in these cells in the early 1990s. Not only have analogues for all major cytoskeletal proteins in eukaryotes been found in prokaryotes, cytoskeletal proteins with no known eukaryotic homologues have also been discovered. Cytoskeletal elements play essential roles in cell division, protection, shape determination, and polarity determination in various prokaryotes.
Tektins are cytoskeletal proteins found in cilia and flagella as structural components of outer doublet microtubules. They are also present in centrioles and basal bodies. They are polymeric in nature, and form filaments.
Alpha-keratin, or α-keratin, is a type of keratin found in vertebrates. This protein is the primary component in hairs, horns, mammalian claws, nails and the epidermis layer of the skin. α-keratin is a fibrous structural protein, meaning it is made up of amino acids that form a repeating secondary structure. The secondary structure of α-keratin is very similar to that of a traditional protein α-helix and forms a coiled coil. Due to its tightly wound structure, it can function as one of the strongest biological materials and has various functions in mammals, from predatory claws to hair for warmth. α-keratin is synthesized through protein biosynthesis, utilizing transcription and translation, but as the cell matures and is full of α-keratin, it dies, creating a strong non-vascular unit of keratinized tissue.
Autumnal equinox or variations, may refer to:
Spring equinox or vernal equinox or variations may refer to:








