Related Research Articles

Estradiol acetate (EA), sold under the brand names Femtrace, Femring, and Menoring, is an estrogen medication which is used in hormone therapy for the treatment of menopausal symptoms in women. It is taken by mouth once daily or given as a vaginal ring once every three months.
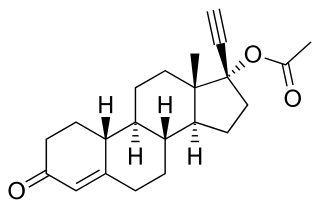
Norethisterone acetate (NETA), also known as norethindrone acetate and sold under the brand name Primolut-Nor among others, is a progestin medication which is used in birth control pills, menopausal hormone therapy, and for the treatment of gynecological disorders. The medication available in low-dose and high-dose formulations and is used alone or in combination with an estrogen. It is ingested orally.

Norgestimate, sold under the brand names Ortho Tri-Cyclen and Previfem among others, is a progestin medication which is used in birth control pills for women and in menopausal hormone therapy. The medication is available in combination with an estrogen and is not available alone. It is taken by mouth.

An estrogen patch is a transdermal delivery system for estrogens such as estradiol and ethinylestradiol which can be used in menopausal hormone therapy, feminizing hormone therapy for transgender women, hormonal birth control, and other uses. Transdermal preparations of estrogen are metabolized differently than oral preparations. Transdermal estrogens avoid the first pass through the liver and thus potentially reduce the risk of blood clotting and stroke.

Bazedoxifene, used as bazedoxifene acetate, is a medication for bone problems and possibly for cancer. It is a third-generation selective estrogen receptor modulator (SERM). Since late 2013 it has had U.S. FDA approval for bazedoxifene as part of the combination drug Duavee in the prevention of postmenopausal osteoporosis. It is also being studied for possible treatment of breast cancer and pancreatic cancer.
Ethinylestradiol/norethisterone acetate (EE/NETA), or ethinylestradiol/norethindrone acetate, is a combination of ethinylestradiol (EE) and norethisterone acetate (NETA) which is used as birth control and menopausal hormone therapy. EE is an estrogen, while norethisterone acetate (NETA) is a progestin. It is taken by mouth. Some preparations of EE/NETA used in birth control additionally contain an iron supplement in the form of ferrous fumarate.
Combined injectable contraceptives (CICs) are a form of hormonal birth control for women. They consist of monthly injections of combined formulations containing an estrogen and a progestin to prevent pregnancy.

Medroxyprogesterone acetate (MPA), also known as depot medroxyprogesterone acetate (DMPA) in injectable form and sold under the brand name Depo-Provera among others, is a hormonal medication of the progestin type. It is used as a method of birth control and as a part of menopausal hormone therapy. It is also used to treat endometriosis, abnormal uterine bleeding, abnormal sexuality in males, and certain types of cancer. The medication is available both alone and in combination with an estrogen. It is taken by mouth, used under the tongue, or by injection into a muscle or fat.

Estradiol cypionate (EC), sold under the brand name Depo-Estradiol among others, is an estrogen medication which is used in hormone therapy for menopausal symptoms and low estrogen levels in cis women, in hormone therapy for trans women, and in hormonal birth control for cis women. It is given by injection into muscle once every 1 to 4 weeks.

Esterified estrogens (EEs), sold under the brand names Estratab and Menest among others, is an estrogen medication which is used hormone therapy for menopausal symptoms and low sex hormone levels in women, to treat breast cancer in both women and men, and to treat prostate cancer in men. It is formulated alone or in combination with methyltestosterone. It is taken by mouth.

Conjugated estrogens (CEs), or conjugated equine estrogens (CEEs), sold under the brand name Premarin among others, is an estrogen medication which is used in menopausal hormone therapy and for various other indications. It is a mixture of the sodium salts of estrogen conjugates found in horses, such as estrone sulfate and equilin sulfate. CEEs are available in the form of both natural preparations manufactured from the urine of pregnant mares and fully synthetic replications of the natural preparations. They are formulated both alone and in combination with progestins such as medroxyprogesterone acetate. CEEs are usually taken by mouth, but can also be given by application to the skin or vagina as a cream or by injection into a blood vessel or muscle.

Estradiol pivalate, also known as estradiol trimethyl acetate (E2-TMA) and sold under the brand name Estrotate, is an estrogen medication and an estrogen ester; specifically, a pivalic acid ester of estradiol. Literature sources are conflicting as to whether the ester is located at the C3 position or at the C17β position. It was marketed as an oil solution for intramuscular injection in the 1940s and 1950s. A combination of estradiol pivalate (1 mg/mL) and progesterone (10 mg/mL) in oil solution for intramuscular injection was available in 1949. An aqueous suspension of estradiol pivalate was also developed by 1950 although whether it was ever marketed is unclear.

Nomegestrol acetate/estradiol (NOMAC-E2), sold under the brand names Naemis and Zoely among others, is a fixed-dose combination medication of nomegestrol acetate, a progestogen, and estradiol, an estrogen, which is used in menopausal hormone therapy and as a birth control pill to prevent pregnancy in women. It is taken by mouth.

Estradiol (E2) is a medication and naturally occurring steroid hormone. It is an estrogen and is used mainly in menopausal hormone therapy and to treat low sex hormone levels in women. It is also used in hormonal birth control for women, in hormone therapy for transgender women, and in the treatment of hormone-sensitive cancers like prostate cancer in men and breast cancer in women, among other uses. Estradiol can be taken by mouth, held and dissolved under the tongue, as a gel or patch that is applied to the skin, in through the vagina, by injection into muscle or fat, or through the use of an implant that is placed into fat, among other routes.

Estradiol cypionate/medroxyprogesterone acetate (EC/MPA), sold under the brand name Cyclofem among others, is a form of combined injectable birth control. It contains estradiol cypionate (EC), an estrogen, and medroxyprogesterone acetate (MPA), a progestin. It is recommended for short-term use and is given once a month by injection into a muscle.

Estradiol/medroxyprogesterone acetate (E2/MPA), sold under the brand names Indivina and Tridestra among others, is a combination product of estradiol, an estrogen, and medroxyprogesterone acetate, a progestogen, which is used in menopausal hormone therapy for the treatment of menopausal symptoms. It is taken by mouth.

Estradiol/dydrogesterone (E2/DYD), sold under the brand name Femoston among others, is a combination of estradiol (E2), an estrogen, and dydrogesterone (DYD), a progestin, which is used in menopausal hormone therapy, specifically to treat and prevent hot flashes and osteoporosis, in postmenopausal women. It is taken by mouth and contains 0.5, 1, or 2 mg E2 and 2.5, 5, 10, or 20 mg DYD per tablet. The medication is marketed widely throughout the world. It is not available in the United States or Canada.
Relugolix/estradiol/norethisterone acetate (RGX/E2/NETA), sold under the brand names Myfembree and Ryeqo, is a fixed-dose combination hormonal medication which is used for the treatment of heavy menstrual bleeding associated with uterine leiomyomas (fibroids) and for moderate to severe pain associated with endometriosis. It contains relugolix (RGX), an orally active gonadotropin-releasing hormone antagonist, estradiol (E2), an estrogen, and norethisterone acetate (NETA), a progestin. The medication is taken by mouth.
References
- ↑ "Conjugated estrogens / medroxyprogesterone Use During Pregnancy". Drugs.com. 16 February 2019. Retrieved 5 May 2020.
- ↑ "Premique Low Dose 0.3mg/1.5mg Modified Release Tablets - Summary of Product Characteristics (SmPC)". (emc). 1 November 2016. Retrieved 5 May 2020.
- ↑ Hochadel M, Avorn J (1 January 2007). The AARP Guide to Pills: Essential Information on More Than 1,200 Prescription and Nonprescription Medications, Including Generics. Sterling Publishing Company Incorporated. pp. 235–. ISBN 978-1-4027-4446-4.
- ↑ "Conjugated Estrogens; Medroxyprogesterone - Drug Usage Statistics". ClinCalc. Retrieved 7 October 2022.