Related Research Articles
Osteopathic medicine is a branch of the medical profession in the United States that promotes the practice of science-based medicine, often referred to in this context as allopathic medicine, with a set of philosophy and principles set by its earlier form, osteopathy. Osteopathic physicians (DOs) are licensed to practice medicine and surgery in all 50 US states. Only graduates of American osteopathic medical colleges may practice the full scope of medicine and surgery generally considered to be medicine by the general public; US DO graduates have historically applied for medical licensure in 87 countries outside of the United States, 85 of which provided them with the full scope of medical and surgical practice. The field is distinct from osteopathic practices offered in nations outside of the U.S., whose practitioners are generally not considered part of core medical staff nor of medicine itself. The other major branch of medicine in the United States is referred to by practitioners of osteopathic medicine as allopathic medicine.

A prescription drug is a pharmaceutical drug that is only permitted to be dispensed to those with a medical prescription. In contrast, over-the-counter drugs can be obtained without a prescription. The reason for this difference in substance control is the potential scope of misuse, from drug abuse to practicing medicine without a license and without sufficient education. Different jurisdictions have different definitions of what constitutes a prescription drug.

Geriatrics, or geriatric medicine, is a medical specialty focused on providing care for the unique health needs of older adults. The term geriatrics originates from the Greek γέρων geron meaning "old man", and ιατρός iatros meaning "healer". It aims to promote health by preventing, diagnosing and treating disease in older adults. There is no defined age at which patients may be under the care of a geriatrician, or geriatric physician, a physician who specializes in the care of elderly people. Rather, this decision is guided by individual patient need and the caregiving structures available to them. This care may benefit those who are managing multiple chronic conditions or experiencing significant age-related complications that threaten quality of daily life. Geriatric care may be indicated if caregiving responsibilities become increasingly stressful or medically complex for family and caregivers to manage independently.
Many countries have measures in place to limit advertising by pharmaceutical companies.

Off-label use is the use of pharmaceutical drugs for an unapproved indication or in an unapproved age group, dosage, or route of administration. Both prescription drugs and over-the-counter drugs (OTCs) can be used in off-label ways, although most studies of off-label use focus on prescription drugs.
The SOAP note is a method of documentation employed by healthcare providers to write out notes in a patient's chart, along with other common formats, such as the admission note. Documenting patient encounters in the medical record is an integral part of practice workflow starting with appointment scheduling, patient check-in and exam, documentation of notes, check-out, rescheduling, and medical billing. Additionally, it serves as a general cognitive framework for physicians to follow as they assess their patients.
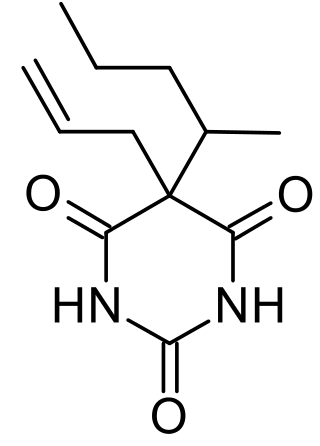
Secobarbital is a short-acting barbiturate derivative drug that was patented in 1934 in the United States. It possesses anaesthetic, anticonvulsant, anxiolytic, sedative, and hypnotic properties. In the United Kingdom, it was known as quinalbarbitone. It is the most frequently used drug in physician-assisted suicide within the United States. Secobarbital is considered to be an obsolete sedative-hypnotic, and as a result, it has largely been replaced by the benzodiazepine family. Seconal was widely abused, known on the street as "red devils" or "reds".
Medication discontinuation is the ceasing of a medication treatment for a patient by either the clinician or the patient themself. When initiated by the clinician, it is known as deprescribing. Medication discontinuation is an important medical practice that may be motivated by a number of reasons:
Sidney R. Garfield was an American medical doctor and a pioneer of health maintenance organizations. He co-founded the Kaiser Permanente healthcare system with businessman Henry J. Kaiser. He graduated from the University of Iowa College of Medicine in 1928, which is now called the Roy J. and Lucille A. Carver College of Medicine.
Academic detailing is "university or non-commercial-based educational outreach." The process involves face-to-face education of prescribers by trained health care professionals, typically pharmacists, physicians, or nurses. The goal of academic detailing is to improve prescribing of targeted drugs to be consistent with medical evidence from randomized controlled trials, which ultimately improves patient care and can reduce health care costs. A key component of non-commercial or university-based academic detailing programs is that they do not have any financial links to the pharmaceutical industry.
Direct-to-consumer advertising (DTCA) refers to the marketing and advertising of pharmaceutical products directly to consumers as patients, as opposed to specifically targeting health professionals. The term is synonymous primarily with the advertising of prescription medicines via mass media platforms—most commonly on television and in magazines, but also via online platforms.

The National Physicians Alliance (NPA) was a 501(c)(3) national, multi-specialty medical organization founded in 2005. The organization's mission statement was: "The National Physicians Alliance creates research and education programs that promote health and foster active engagement of physicians with their communities to achieve high quality, affordable health care for all. The NPA offers a professional home to physicians across medical specialties who share a commitment to professional integrity and health justice." In 2019, they merged with Doctors for America.
A seeding trial or marketing trial is a form of marketing, conducted in the name of research, designed to target product sampling towards selected consumers. In the marketing research field, seeding is the process of allocating marketing to specific customers, or groups of customers, in order to stimulate the internal dynamics of the market, and enhance the diffusion process. In medicine, seeding trials are clinical trials or research studies in which the primary objective is to introduce the concept of a particular medical intervention—such as a pharmaceutical drug or medical device—to physicians, rather than to test a scientific hypothesis.
David Franklin is an American microbiologist and former fellow of Harvard Medical School who while employed by Parke-Davis filed the 1996 whistleblower lawsuit exposing their illegal promotion of Neurontin (gabapentin) for off-label uses. Franklin's suit, filed on behalf of the citizens of the United States under the qui tam provisions of US federal and state law, uncovered illegal pharmaceutical industry practices and created new legal precedent that resulted in a cascade of criminal convictions and civil and criminal penalties against Pfizer and several other pharmaceutical companies totalling more than $7 billion. Civil cases also followed Franklin v. Parke-Davis. Insurance companies, led by Kaiser Permanente, sued Pfizer for fraud and violation of the federal Racketeer Influenced and Corrupt Organizations Act; the Kaiser case settled in April 2014 after Pfizer's appeal at the US Supreme Court was rejected. Franklin v. Pfizer also spawned more than a thousand wrongful death (suicide) suits associated with use of Neurontin. Numerous books have addressed the social, economic and healthcare implications of Dr. Franklin's stance and actions. The settlement was the first off-label promotion settlement under the False Claims Act.
PharmedOut (PhO) is a Georgetown University Medical Center project founded in 2006. It is directed by Adriane Fugh-Berman. The stated mission of the organization is to advance evidence-based prescribing and educate healthcare professionals about pharmaceutical marketing practices. Stated goals are to: 1. Document and disseminate information about how pharmaceutical companies influence prescribing 2. Foster access to unbiased information about drugs and 3. Encourage physicians to choose pharma-free CME.
Sorrell v. IMS Health Inc., 564 U.S. 552 (2011), is a United States Supreme Court case in which the Court held that a Vermont statute that restricted the sale, disclosure, and use of records that revealed the prescribing practices of individual doctors violated the First Amendment.

Franklin v. Parke-Davis is a lawsuit filed in 1996 against Parke-Davis, a division of Warner-Lambert Company, and eventually against Pfizer under the qui tam provisions of the False Claims Act. The suit was commenced by David Franklin, a microbiologist hired in the spring of 1996 in a sales capacity at Parke-Davis, a pharmaceutical subsidiary of Warner-Lambert. In denying the defendants' motion for summary judgment, the court for the first time recognized off-label promotion of drugs could cause Medicaid to pay for prescriptions that were not reimbursable, triggering False Claims Act liability. The case was also significant in exposing the degree to which publication bias impacts the randomized controlled studies conducted by pharmaceutical companies to test the efficacy of their products. Ultimately, the parties reached a settlement agreement of $430 million to resolve all civil claims and criminal charges stemming from the qui tam complaint. At the time of the settlement in May 2004, it represented one of the largest False Claims Act recoveries against a pharmaceutical company in U.S. history, and was the first off-label promotion settlement under the False Claims Act.
Adriane Fugh-Berman is a professor in the department of pharmacology and physiology, and in the department of family medicine, at Georgetown University Medical Center. She is also the director of PharmedOut, a Georgetown University Medical Center project that promotes rational prescribing and researches the effects of pharmaceutical and medical device industry marketing on prescribing behavior and therapeutic choices. Additionally, she is the Co-Director of the M.S. in Health and the Public Interest (HAPI) Program at the Georgetown University Graduate School of Arts & Sciences.
Diethylstilbestrol dipalmitate, also known as stilpalmitate, is a synthetic, nonsteroidal estrogen of the stilbestrol group and an ester of diethylstilbestrol (DES) that was formerly marketed but is now no longer available. Its actions and uses are essentially the same as those of DES, but it is absorbed more slowly and for this reason has a much longer duration of action and improved tolerability in comparison. A single 5 mg intramuscular injection of DES dipalmitate in oil solution has been found to have an average duration of action of 8 to 10 weeks in terms of relief of menopausal symptoms, with a duration of as long as 15 to 16 weeks occurring in some women. A single 15 or 20 mg intramuscular injection of DES dipalmitate in oil solution will control menopausal symptoms for 3 months or longer. DES dipalmitate in aqueous suspension by intramuscular injection has been studied as well.

In the United States, the opioid epidemic is an extensive ongoing overuse of opioid medications, both from medical prescriptions and illegal sources. The epidemic began in the United States in the late 1990s, according to the Centers for Disease Control and Prevention (CDC), when opioids were increasingly prescribed for pain management, resulting in a rise in overall opioid use throughout subsequent years. The great majority of Americans who use prescription opioids do not believe that they are misusing them.
References
- ↑ Rutkow, Lainie; Teret, Stephen (October 2010). "The Potential for State Attorneys General to Promote the Public's Health: Theory, Evidence, and Practice". Robert Wood Johnson Foundation Public Health Law Research Program, via FOLIO.
- ↑ Price, DW; Raebel, MA; Conner, DA; Wright, LA (2008). "Prescribers' and Organizational Leaders' Preferences for Education about Heavily Marketed Drugs". The Permanente Journal. 12 (2): 28–35. doi:10.7812/tpp/07-106. PMC 3042287 . PMID 21364809.
- ↑ "Attorney Generals' Prescriber and Consumer Education Grant". College of Pharmacy. 4 October 2011.
- ↑ Price, DW; Raebel, MA; Conner, DA; Wright, LA (2008). "Prescribers' and Organizational Leaders' Preferences for Education about Heavily Marketed Drugs". The Permanente Journal. 12 (2): 28–35. doi:10.7812/tpp/07-106. PMC 3042287 . PMID 21364809.
- ↑ Elliott, Carl (22 May 2012). "Pharmed Out: an Interview With Adriane Fugh-Berman". The Chronicle of Higher Education Blogs: Brainstorm.
- ↑ "Federation of State Medical Boards". www.fsmb.org. Archived from the original on 2016-05-20. Retrieved 2017-06-21.
- ↑ Vermont, University of (14 April 2006). "University Communications : University of Vermont". www.uvm.edu.
- ↑ Nachbur, Jennifer (May 21, 2013). "Advancing Integrity in Medical Education". www.pewtrusts.org. The Pew Charitable Trusts.
- ↑ Krautkramer, Christian J. (June 2006). "Neurontin and off-label marketing". AMA Journal of Ethics . 8 (6): 397–402. doi: 10.1001/virtualmentor.2006.8.6.hlaw1-0606 . PMID 23234671.