| Look up coupling in Wiktionary, the free dictionary. |
Coupling is a connection or joint between two things.

A coupling is a device used to connect two shafts together at their ends for the purpose of transmitting power. The primary purpose of couplings is to join two pieces of rotating equipment while permitting some degree of misalignment or end movement or both. In a more general context, a coupling can also be a mechanical device that serves to connect the ends of adjacent parts or objects. Couplings do not normally allow disconnection of shafts during operation, however there are torque limiting couplings which can slip or disconnect when some torque limit is exceeded. Selection, installation and maintenance of couplings can lead to reduced maintenance time and maintenance cost.
Coupling may also refer to:
- Coupling (physics), when two systems are interacting with each other
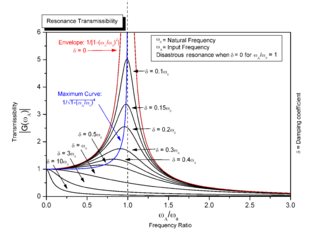
- Rotational–vibrational coupling, occurring when rotation frequency of an object is close to or identical to a natural internal vibration frequency
- Angular momentum coupling, the combining of quantized angular momentum (e.g., the interaction between two nuclei in nuclear magnetic resonance)
- Quantum coupling, when quantum states in one of the systems will cause an instantaneous change in all of the bound systems
Quantum Coupling is an effect in quantum mechanics in which two or more quantum systems are bound such that a change in one of the quantum states in one of the systems will cause an instantaneous change in all of the bound systems. It is a state similar to quantum entanglement but whereas quantum entanglement can take place over long distances quantum coupling is restricted to quantum scales.
- Coupling (computer programming), the degree to which each program module relies on each one of the other modules
- Coupling (electronics), the transfer of a signal from one medium or circuit block to another
- Coupling (genetics), a type of genetic linkage
- Coupling (piping), a short length of pipe or tube to connect two pipes or tubes together
- Coupling (probability), a proof technique in probability theory
- Railway coupling, a mechanism for connecting railway rolling stock
- Azo coupling, often called "coupling", an electrophilic substitution reaction
- Coupling reaction, reactions between hydrocarbon fragments in organic chemistry
- Hose coupling, a piece on the end of a hose to connect it to extra hoses or hose appliances
- Coupling track, a term in music recording for a B-side track
- Joint encoding, an audio compression technique in which the redundancy of information between audio channels is reduced; also commonly known as channel coupling or stereo coupling
- Mating or the act of sexual intercourse
- Coupling, the distance formed by the lumbar vertebrae of a horse's back.
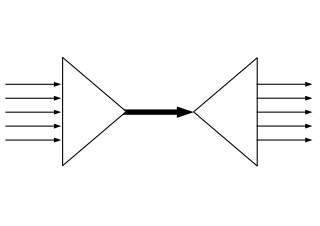
In physics, two objects are said to be coupled when they are interacting with each other. In classical mechanics, coupling is a connection between two oscillating systems, such as pendulums connected by a string. The connection affects the oscillatory pattern of both objects. In particle physics, two particles are coupled if they are connected by one of the four fundamental forces.

Rotational–vibrational coupling occurs when the rotation frequency of an object is close to or identical to a natural internal vibration frequency. The animation on the right shows a simple example. The motion depicted in the animation is for the idealized situation that the force exerted by the spring increases linearly with the distance to the center of rotation. Also, the animation depicts what would occur if there would not be any friction.
In quantum mechanics, the procedure of constructing eigenstates of total angular momentum out of eigenstates of separate angular momenta is called angular momentum coupling. For instance, the orbit and spin of a single particle can interact through spin–orbit interaction, in which case the complete physical picture must include spin-orbit coupling. Or two charged particles, each with a well-defined angular momentum, may interact by Coulomb forces, in which case coupling of the two one-particle angular momenta to a total angular momentum is a useful step in the solution of the two-particle Schrödinger equation. In both cases the separate angular momenta are no longer constants of motion, but the sum of the two angular momenta usually still is. Angular momentum coupling in atoms is of importance in atomic spectroscopy. Angular momentum coupling of electron spins is of importance in quantum chemistry. Also in the nuclear shell model angular momentum coupling is ubiquitous.