Related Research Articles
Cytomegalovirus (CMV) is a genus of viruses in the order Herpesvirales, in the family Herpesviridae, in the subfamily Betaherpesvirinae. Humans and other primates serve as natural hosts. The 11 species in this genus include human betaherpesvirus 5, which is the species that infects humans. Diseases associated with HHV-5 include mononucleosis and pneumonia, and congenital CMV in infants can lead to deafness and ambulatory problems.

SV40 is an abbreviation for simian vacuolating virus 40 or simian virus 40, a polyomavirus that is found in both monkeys and humans. Like other polyomaviruses, SV40 is a DNA virus that sometimes causes tumors in animals, but most often persists as a latent infection. SV40 has been widely studied as a model eukaryotic virus, leading to many early discoveries in eukaryotic DNA replication and transcription.

Kaposi's sarcoma-associated herpesvirus (KSHV) is the ninth known human herpesvirus; its formal name according to the International Committee on Taxonomy of Viruses (ICTV) is Human gammaherpesvirus 8, or HHV-8 in short. Like other herpesviruses, its informal names are used interchangeably with its formal ICTV name. This virus causes Kaposi's sarcoma, a cancer commonly occurring in AIDS patients, as well as primary effusion lymphoma, HHV-8-associated multicentric Castleman's disease and KSHV inflammatory cytokine syndrome. It is one of seven currently known human cancer viruses, or oncoviruses. Even after many years since the discovery of KSHV/HHV8, there is no known cure for KSHV associated tumorigenesis.

An arenavirus is a bi- or trisegmented ambisense RNA virus that is a member of the family Arenaviridae. These viruses infect rodents and occasionally humans. A class of novel, highly divergent arenaviruses, properly known as reptarenaviruses, have also been discovered which infect snakes to produce inclusion body disease, mostly in boa constrictors. At least eight arenaviruses are known to cause human disease. The diseases derived from arenaviruses range in severity. Aseptic meningitis, a severe human disease that causes inflammation covering the brain and spinal cord, can arise from the lymphocytic choriomeningitis virus. Hemorrhagic fever syndromes, including Lassa fever, are derived from infections such as Guanarito virus, Junin virus, Lassa virus, Lujo virus, Machupo virus, Sabia virus, or Whitewater Arroyo virus. Because of the epidemiological association with rodents, some arenaviruses and bunyaviruses are designated as roboviruses.
Alphavirus is a genus of RNA viruses, the sole genus in the Togaviridae family. Alphaviruses belong to group IV of the Baltimore classification of viruses, with a positive-sense, single-stranded RNA genome. There are 32 alphaviruses, which infect various vertebrates such as humans, rodents, fish, birds, and larger mammals such as horses, as well as invertebrates. Alphaviruses that could infect both vertebrates and arthropods are referred dual-host alphaviruses, while insect-specific alphaviruses such as Eilat virus and Yada yada virus are restricted to their competent arthropod vector. Transmission between species and individuals occurs mainly via mosquitoes, making the alphaviruses a member of the collection of arboviruses – or arthropod-borne viruses. Alphavirus particles are enveloped, have a 70 nm diameter, tend to be spherical, and have a 40 nm isometric nucleocapsid.

Tomato bushy stunt virus (TBSV) is a virus of the tombusvirus family. It was first reported in tomatoes in 1935 and primarily affects vegetable crops, though it is not generally considered an economically significant plant pathogen. Depending upon the host, TBSV causes stunting of growth, leaf mottling, and deformed or absent fruit. The virus is likely to be soil-borne in the natural setting, but can also be transmitted mechanically, for example through contaminated cutting tools. TBSV has been used as a model system in virology research on the life cycle of plant viruses, particularly in experimental infections of the model host plant Nicotiana benthamiana.

Human herpesvirus 6 (HHV-6) is the common collective name for human betaherpesvirus 6A (HHV-6A) and human betaherpesvirus 6B (HHV-6B). These closely related viruses are two of the nine known herpesviruses that have humans as their primary host.

Herpesviridae is a large family of DNA viruses that cause infections and certain diseases in animals, including humans. The members of this family are also known as herpesviruses. The family name is derived from the Greek word ἕρπειν, referring to spreading cutaneous lesions, usually involving blisters, seen in flares of herpes simplex 1, herpes simplex 2 and herpes zoster (shingles). In 1971, the International Committee on the Taxonomy of Viruses (ICTV) established Herpesvirus as a genus with 23 viruses among four groups. As of 2020, 115 species are recognized, all but one of which are in one of the three subfamilies. Herpesviruses can cause both latent and lytic infections.

Duck plague is a worldwide disease caused by Anatid alphaherpesvirus 1 (AnHV-1) of the family Herpesviridae that causes acute disease with high mortality rates in flocks of ducks, geese, and swans. It is spread both vertically and horizontally—through contaminated water and direct contact. Migratory waterfowl are a major factor in the spread of this disease as they are often asymptomatic carriers of disease. The incubation period is three to seven days. Birds as young as one week old can be infected. DEV is not zoonotic.

Gammaherpesvirinae is a subfamily of viruses in the order Herpesvirales and in the family Herpesviridae. Viruses in Gammaherpesvirinae are distinguished by reproducing at a more variable rate than other subfamilies of Herpesviridae. Mammals serve as natural hosts. There are 43 species in this subfamily, divided among 7 genera with three species unassigned to a genus. Diseases associated with this subfamily include: HHV-4: infectious mononucleosis. HHV-8: Kaposi's sarcoma.
Turnip crinkle virus (TCV) is a plant pathogenic virus of the family Tombusviridae. It was first isolated from turnip.
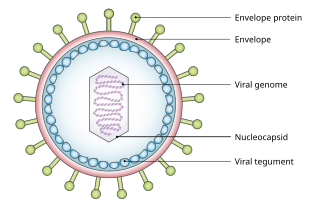
A viral tegument or tegument, more commonly known as a viral matrix, is a cluster of proteins that lines the space between the envelope and nucleocapsid of all herpesviruses. The tegument generally contains proteins that aid in viral DNA replication and evasion of the immune response, typically with inhibition of signalling in the immune system and activation of interferons. The tegument is usually released shortly after infection into the cytoplasm. These proteins are usually formed within the late phase of the viral infectious cycle, after viral genes have been replicated. Much information regarding viral teguments has been gathered from studying herpes simplex virus.

Neutral alpha-glucosidase AB is an enzyme that in humans is encoded by the GANAB gene.

Mannosyl-oligosaccharide glucosidase is an enzyme that in humans is encoded by the MOGS gene.

Herpesvirus glycoprotein B is a viral glycoprotein that is involved in the viral cell entry of Herpes simplex virus (HSV). Herpesviruses have a lipid bilayer, called the envelope, which contains twelve surface glycoproteins. For infectivity to be attained, the double stranded DNA genome of HSV must enter the host cell through means of fusion of its envelope with the cellular membrane or via endocytosis. Other viral glycoproteins involved in the process of viral cell entry include gC, gB, gD, gH, and gL, but only gC, gB, gD, and gH are required for the fusion of the HSV's envelope with the cellular membrane. It can be noted that all herpesviruses have glycoproteins gB, gH, and gL.
Peter K. Vogt is an American molecular biologist, virologist and geneticist. His research focuses on retroviruses and viral and cellular oncogenes.
HSV epigenetics is the epigenetic modification of herpes simplex virus (HSV) genetic code.
Cyvirus cyprinidallo1, also known as Cyprinid herpesvirus 1 (CyHV-1) is a species of virus in the genus Cyprinivirus, family Alloherpesviridae, and order Herpesvirales.
Ictalurid herpesvirus 1 (IcHV-1) is a species of virus in the genus Ictalurivirus, family Alloherpesviridae, and order Herpesvirales. It causes disease in channel catfish and blue catfish, and can cause significant economic loss in catfish farms. The disease is endemic in the USA and there are reports of the virus in Honduras and Russia.
Caprine alphaherpesvirus 1 (CpHV-1) is a species of virus known to infect goats worldwide. It has been shown to produce systemic and respiratory symptoms in kids and to cause abortions in adult goats.
References
- 1 2 3 4 5 "Dennis J. O'Callaghan, PhD" (PDF). News and Events, Graduate School, University of Mississippi Medica Center. 2008.
- ↑ Catalog of Copyright Entries. Third Series: 1969: January-June. Copyright Office, Library of Congress. 1972. p. 826.
- ↑ "Obituary. Dr. Charles Chandler Randall". Clarion Ledger. Jackson, Mississippi. April 4, 2007.
- 1 2 "Dr. Dennis O'Callaghan retires after 34 years at LSU Health Shreveport | BIZ - Northwest Louisiana". March 18, 2018.
- ↑ Official Gazette of the United States Patent and Trademark Office: Patents. U.S. Department of Commerce, Patent and Trademark Office. 1998. p. 2756.
- ↑ "Professor Nikolaus Osterrieder". Jockey Club College of Veterinary Medicine and Life Sciences, City University of Hong Kong.
- ↑ Granoff, Allan; Webster, Robert G., eds. (27 July 1999). "Equine herpesviruses (Herpesviridae) by Dennis J. O'Callaghan and Nikolaus Osterrieder". Encyclopedia of Virology. Elsevier. pp. 508–515. ISBN 9780080547978.
- ↑ "Historic Fellows". American Association for the Advancement of Science.
- ↑ "Helen Frances Briscoe O'Callaghan 1941–2018". The Shreveport Times. Shreveport, Louisiana. November 28, 2018.