Related Research Articles
An allergen is a type of antigen that produces an abnormally vigorous immune response in which the immune system fights off a perceived threat that would otherwise be harmless to the body. Such reactions are called allergies.

Pseudoephedrine (PSE) is a sympathomimetic drug of the phenethylamine and amphetamine chemical classes. It may be used as a nasal/sinus decongestant, as a stimulant, or as a wakefulness-promoting agent in higher doses.

Tylenol is a brand of medication, advertised for reducing pain, reducing fever, and relieving the symptoms of allergies, cold, cough, headache, and influenza. The active ingredient of its original flagship product is paracetamol, an analgesic and antipyretic. Like the words paracetamol and acetaminophen, the brand name Tylenol is derived from a chemical name for the compound, N-acetyl-para-aminophenol (APAP). The brand name is owned by McNeil Consumer Healthcare, a subsidiary of Kenvue.

Ephedrine is a central nervous system (CNS) stimulant that is often used to prevent low blood pressure during anesthesia. It has also been used for asthma, narcolepsy, and obesity but is not the preferred treatment. It is of unclear benefit in nasal congestion. It can be taken by mouth or by injection into a muscle, vein, or just under the skin. Onset with intravenous use is fast, while injection into a muscle can take 20 minutes, and by mouth can take an hour for effect. When given by injection it lasts about an hour and when taken by mouth it can last up to four hours.

Over-the-counter (OTC) drugs are medicines sold directly to a consumer without a requirement for a prescription from a healthcare professional, as opposed to prescription drugs, which may be supplied only to consumers possessing a valid prescription. In many countries, OTC drugs are selected by a regulatory agency to ensure that they contain ingredients that are safe and effective when used without a physician's care. OTC drugs are usually regulated according to their active pharmaceutical ingredient (API) rather than final products. By regulating APIs instead of specific drug formulations, governments allow manufacturers the freedom to formulate ingredients, or combinations of ingredients, into proprietary mixtures.

Xylometazoline, also spelled xylomethazoline, is a medication used to reduce symptoms of nasal congestion, allergic rhinitis, and sinusitis. Use is not recommended for more than seven days. Use is also not recommended in those less than three months of age and some say not less than 6 years of age. It is used directly in the nose as a spray or drops.

Cold medicines are a group of medications taken individually or in combination as a treatment for the symptoms of the common cold and similar conditions of the upper respiratory tract. The term encompasses a broad array of drugs, including analgesics, antihistamines and decongestants, among many others. It also includes drugs which are marketed as cough suppressants or antitussives, but their effectiveness in reducing cough symptoms is unclear or minimal.

Loratadine, sold under the brand name Claritin among others, is a medication used to treat allergies. This includes allergic rhinitis and hives. It is also available in drug combinations such as loratadine/pseudoephedrine, in which it is combined with pseudoephedrine, a nasal decongestant. It is taken orally.
A decongestant, or nasal decongestant, is a type of pharmaceutical drug that is used to relieve nasal congestion in the upper respiratory tract. The active ingredient in most decongestants is either pseudoephedrine or phenylephrine. Intranasal corticosteroids can also be used as decongestants and antihistamines can be used to alleviate runny nose, nasal itch, and sneezing.
Vicks NyQuil is a brand of over-the-counter medication manufactured by Procter & Gamble intended for the relief of various symptoms of the common cold. All medications within the NyQuil imprint contain sedating antihistamines, hypnotics, and/or alcohol, and are intended to be taken before sleep. Its daytime counterpart is antihistamine-free DayQuil, formulated to avoid drowsiness. NyQuil is also used as a sleep aid. NyQuil was first marketed in the United States in 1966.
Schering-Plough Corporation was an American pharmaceutical company. It was originally the U.S. subsidiary of the German company Schering AG, which was founded in 1851 by Ernst Christian Friedrich Schering. As a result of nationalization, it became an independent company. In 1971, the Schering Corporation merged with Plough, Inc. to form Schering-Plough. On November 4, 2009 Merck & Co. merged with Schering-Plough with the new company taking the name of Merck & Co.

Benadryl is a brand of various antihistamine medications used to stop allergies, whose content varies in different countries, but which includes some combination of diphenhydramine, acrivastine, and/or cetirizine.

Propylhexedrine, commonly sold under the brand name Benzedrex, is an alkylamine primarily utilized as a topical nasal decongestant. Its main indications are relief of congestion due to colds, allergies, and allergic rhinitis.

Phenylephrine is a medication used as a decongestant for uncomplicated nasal congestion, used to dilate the pupil, used to increase blood pressure, and used to relieve hemorrhoids. It can be taken by mouth, as a nasal spray, given by injection into a vein or muscle, or applied to the skin.
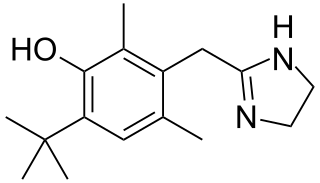
Oxymetazoline, sold under the brand name Afrin among others, is a topical decongestant and vasoconstrictor medication. It is available over-the-counter as a nasal spray to treat nasal congestion and nosebleeds, as eyedrops to treat eye redness due to minor irritation, and as a prescription topical cream to treat persistent facial redness due to rosacea in adults. Its effects begin within minutes and last for up to six hours. Intranasal use for longer than three days may cause congestion to recur or worsen, resulting in physical dependence.
Actifed is a registered trademark for a combination antihistamine and nasal decongestant medication used for cold and allergy symptoms. Actifed was developed in 1958 by Burroughs Wellcome & Company, later Haleon.

The Combat Methamphetamine Epidemic Act of 2005 (CMEA) is federal legislation enacted in the United States on March 9, 2006, to regulate, among other things, retail over-the-counter sales of following products because of their use in the manufacture of illegal drugs:

Methamphetamine in the United States is regulated under Schedule II of the Controlled Substances Act. It is approved for pharmacological use in the treatment of attention deficit hyperactivity disorder, narcolepsy, and treatment-resistant obesity, but it is primarily used as a recreational drug. In 2012, 16,000 prescriptions for methamphetamine were filled, approximately 1.2 million Americans reported using it in the past year, and 440,000 reported using the drug in the past month.
Fexofenadine/pseudoephedrine, sold under the brand name Allegra-D among others, is a fixed-dose combination medication used for the treatment of nasal congestion and other symptoms of allergies and the common cold. It contains fexofenadine, as the hydrochloride, an antihistamine; and pseudoephedrine, as the hydrochloride, a nasal decongestant.
References
- ↑ Payne JW (March 9, 2009). "Drixoral: Why the Allergy Medicine Isn't Available, and What to Use Instead". U.S. News & World Report . Retrieved 2010-02-22.
- ↑ "Products Currently Unavailable". Merck. 2010. Retrieved 2010-05-18.
- ↑ "Product Information:Drixoral". Health Canada. Retrieved 2024-01-12.
- ↑ "1988 Drixoral Commercial". Drixoral. 1988. Retrieved 2021-03-21.