Dihydrofuran may refer to:
| This set index page lists chemical compounds articles associated with the same name. If an internal link led you here, you may wish to change the link to point directly to the intended article. |
Dihydrofuran may refer to:
| This set index page lists chemical compounds articles associated with the same name. If an internal link led you here, you may wish to change the link to point directly to the intended article. |

High-performance liquid chromatography is a technique in analytical chemistry used to separate, identify, and quantify each component in a mixture. It relies on pumps to pass a pressurized liquid solvent containing the sample mixture through a column filled with a solid adsorbent material. Each component in the sample interacts slightly differently with the adsorbent material, causing different flow rates for the different components and leading to the separation of the components as they flow out of the column.
Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen. Chemical compounds containing such rings are also referred to as furans.

Bromo-DragonFLY is a psychedelic drug related to the phenethylamine family. Bromo-DragonFLY is considered a potent hallucinogen, having around one third the potency of LSD with a normal dose in the region of 200 μg to 800 μg, and it has an extremely long duration of action, up to several days. Bromo-DragonFLY has a stereocenter and (R)-(−)-bromo-DragonFLY is the more active stereoisomer.
The Heck reaction is the chemical reaction of an unsaturated halide with an alkene in the presence of a base and a palladium catalyst to form a substituted alkene. It is named after Tsutomu Mizoroki and Richard F. Heck. Heck was awarded the 2010 Nobel Prize in Chemistry, which he shared with Ei-ichi Negishi and Akira Suzuki, for the discovery and development of this reaction. This reaction was the first example of a carbon-carbon bond-forming reaction that followed a Pd(0)/Pd(II) catalytic cycle, the same catalytic cycle that is seen in other Pd(0)-catalyzed cross-coupling reactions. The Heck reaction is a way to substitute alkenes.

Pyridinium chlorochromate (PCC) is a yellow-orange salt with the formula [C5H5NH]+[CrO3Cl]−. It is a reagent in organic synthesis used primarily for oxidation of alcohols to form carbonyls. A variety of related compounds are known with similar reactivity. PCC offers the advantage of the selective oxidation of alcohols to aldehydes or ketones, whereas many other reagents are less selective.
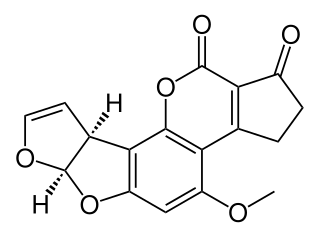
Aflatoxin total synthesis concerns the total synthesis of a group of organic compounds called aflatoxins. These compounds occur naturally in several fungi. As with other chemical compound targets in organic chemistry, the organic synthesis of aflatoxins serves various purposes. Traditionally it served to prove the structure of a complex biocompound in addition to evidence obtained from spectroscopy. It also demonstrates new concepts in organic chemistry and opens the way to molecular derivatives not found in nature. And for practical purposes, a synthetic biocompound is a commercial alternative to isolating the compound from natural resources. Aflatoxins in particular add another dimension because it is suspected that they have been mass-produced in the past from biological sources as part of a biological weapons program.
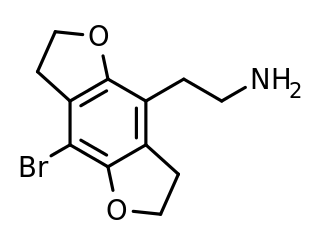
2C-B-FLY is a psychedelic phenethylamine of the 2C family. It was first synthesized in 1996 by Aaron P. Monte.
The molecular formula C4H6O may refer to:

Manganese(III) acetate describes a family of coordination complexes with the approximate formula Mn(O2CCH3)3. All are brown solids, which are soluble in acetic acid and water. These compounds are used in organic synthesis as oxidizing agents.
In enzymology, a muconolactone Delta-isomerase is an enzyme that catalyzes the chemical reaction

Lactarius helvus, commonly known as fenugreek milkcap, is a member of the large milkcap genus Lactarius in the order Russulales. Fruiting bodies can be found in Sphagnum moss in coniferous and deciduous woodland in Europe, and possibly North America, although considerable debate continues about the North American variety, formerly referred to as Lactarius aquifluus. Mushrooms are pale brown-grey or beige in colour and funnel-shaped, with colourless, watery milk. Its distinctive smell has been likened to fenugreek, celery, liquorice, or Maggi instant soup. Mildly toxic when raw, it has been implicated in the poisoning of 418 people near Leipzig in October 1949. However, it is used in small quantities as a spice when dried. Sotolon, the agent that gives the fungus its odour, also occurs in fenugreek, maple syrup and lovage.

The Achmatowicz reaction, also known as the Achmatowicz rearrangement, is an organic synthesis in which a furan is converted to a dihydropyran. In the original publication by the Polish Chemist Osman Achmatowicz Jr. in 1971 furfuryl alcohol is reacted with bromine in methanol to 2,5-dimethoxy-2,5-dihydrofuran which rearranges to the dihydropyran with dilute sulfuric acid. Additional reaction steps, alcohol protection with methyl orthoformate and boron trifluoride) and ketone reduction with sodium borohydride produce an intermediate from which many monosaccharides can be synthesised.

Diisopinocampheylborane is an organoborane that is useful for asymmetric synthesis. This colourless solid is the precursor to a range of related reagents. The compound was reported in 1961 by Zweifel and Brown in a pioneering demonstration of asymmetric synthesis using boranes. The reagent is mainly used for the synthesis of chiral secondary alcohols.
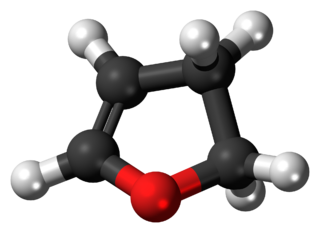
2,3-Dihydrofuran is a heterocyclic compound. It is one of the simplest enol ethers and a position isomer of 2,5-dihydrofuran. It is a colorless volatile liquid.

2,5-Dihydrofuran is an organic compound classified as a monounsaturated derivative of furan. It is a colorless, volatile liquid. It can be produced by the rearrangement of the epoxide of butadiene.

Philip Kraft is a German fragrance chemist. Since 1996 he has been employed by Givaudan, a leading Flavor and Fragrance company, where he designs captive odorants for use in perfumes. He has lectured at the University of Bern, the University of Zurich, and the ETH Zurich.
Manganese-mediated coupling reactions are radical coupling reactions between enolizable carbonyl compounds and unsaturated compounds initiated by a manganese(III) salt, typically manganese(III) acetate. Copper(II) acetate is sometimes used as a co-oxidant to assist in the oxidation of intermediate radicals to carbocations.

3-Hydroxytetrahydrofuran is a colorless liquid with a normal boiling point of 179 °C and boiling at 88−89 °C at 17 mmHg, with density. 3-OH THF is a useful pharmaceutical intermediate. The chiral version of this compound is an intermediate to launched retroviral drugs.

Alexander Latham Dounce was an American professor of biochemistry. Among his fields of study were the isolation and purification of cellular organelles, protein crystallization, enzymes, DNA binding proteins, and the chemical basis of protein synthesis. He also invented the Dounce homogenizer, which was named after him.