
Dioxane may refer to the following chemical compounds:

Dioxane may refer to the following chemical compounds:

Trichloroethylene (TCE) is a carcinogenic halocarbon with the formula C2HCl3, commonly used as an industrial degreasing solvent. It is a clear, colourless non-flammable liquid with a chloroform-like pleasant and pungent smell. The IUPAC name is trichloroethene. Industrial abbreviations include TCE, trichlor, Trike, Tricky and tri. It should not be confused with the similar 1,1,1-trichloroethane, which is commonly known as chlorothene. It has been sold under a variety of trade names. Under the trade names Trimar and Trilene, trichloroethylene was used as a volatile anesthetic and as an inhaled obstetrical analgesic in millions of patients.
Tin(IV) chloride, also known as tin tetrachloride or stannic chloride, is an inorganic compound with the formula SnCl4. It is a colorless hygroscopic liquid, which fumes on contact with air. It is used as a precursor to other tin compounds. It was first discovered by Andreas Libavius (1550–1616) and was known as spiritus fumans libavii.

1,4-Dioxane is a heterocyclic organic compound, classified as an ether. It is a colorless liquid with a faint sweet odor similar to that of diethyl ether. The compound is often called simply dioxane because the other dioxane isomers are rarely encountered.
Dioxolane is a heterocyclic acetal with the chemical formula (CH2)2O2CH2. It is related to tetrahydrofuran (THF) by replacement of the methylene group (CH2) at 2nd position of THF with an oxygen atom. The corresponding saturated 6-membered C4O2 rings are called dioxanes. The isomeric 1,2-dioxolane (wherein the two oxygen centers are adjacent) is a peroxide. 1,3-dioxolane is used as a solvent and as a comonomer in polyacetals.
The molecular formula C4H8O2 may refer to:
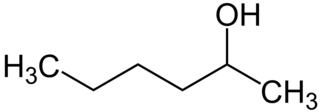
Hexanol may refer to any of the following isomeric organic compounds with the formula C6H13OH:

The Prins reaction is an organic reaction consisting of an electrophilic addition of an aldehyde or ketone to an alkene or alkyne followed by capture of a nucleophile or elimination of an H+ ion. The outcome of the reaction depends on reaction conditions. With water and a protic acid such as sulfuric acid as the reaction medium and formaldehyde the reaction product is a 1,3-diol (3). When water is absent, the cationic intermediate loses a proton to give an allylic alcohol (4). With an excess of formaldehyde and a low reaction temperature the reaction product is a dioxane (5). When water is replaced by acetic acid the corresponding esters are formed.
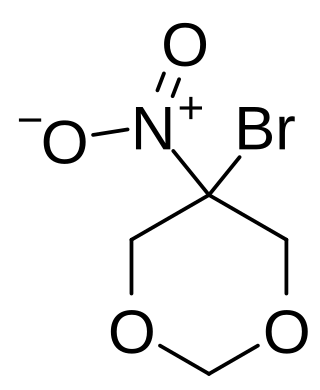
Bronidox, or 5-bromo-5-nitro-1,3-dioxane, is an antimicrobial chemical compound.
The Schlenk equilibrium, named after its discoverer Wilhelm Schlenk, is a chemical equilibrium taking place in solutions of Grignard reagents and Hauser bases
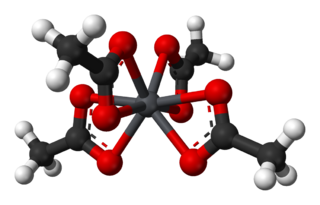
Lead(IV) acetate or lead tetraacetate is an metalorganic compound with chemical formula Pb(C2H3O2)4. It is a colorless solid that is soluble in nonpolar, organic solvents, indicating that it is not a salt. It is degraded by moisture and is typically stored with additional acetic acid. The compound is used in organic synthesis.
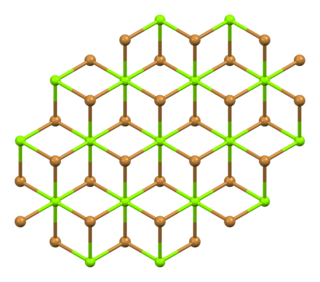
Magnesium bromide is a chemical compound of magnesium and bromine, with the chemical formula MgBr2. It is white and deliquescent crystalline solid. It is often used as a mild sedative and as an anticonvulsant for treatment of nervous disorders. It is water-soluble and somewhat soluble in alcohol. It can be found naturally in small amounts in some minerals such as: bischofite and carnallite, and in sea water, such as that of the Dead Sea.
Methylmagnesium chloride is an organometallic compound with the general formula CH3MgCl. This highly flammable, colorless, and moisture sensitive material is the simplest Grignard reagent and is commercially available, usually as a solution in tetrahydrofuran.
The molecular formula C6H8O4 may refer to:

Germanium dichloride is a chemical compound of germanium and chlorine with the formula GeCl2. It is a yellow solid. Germanium dichloride is an example of a compound featuring germanium in the +2 oxidation state.

Electrical resistance heating (ERH) is an intensive in situ environmental remediation method that uses the flow of alternating current electricity to heat soil and groundwater and evaporate contaminants. Electric current is passed through a targeted soil volume between subsurface electrode elements. The resistance to electrical flow that exists in the soil causes the formation of heat; resulting in an increase in temperature until the boiling point of water at depth is reached. After reaching this temperature, further energy input causes a phase change, forming steam and removing volatile contaminants. ERH is typically more cost effective when used for treating contaminant source areas.
Dioxin may refer to:

Dioxane tetraketone (or 1,4-dioxane-2,3,5,6-tetrone) is an organic compound with the formula C4O6. It is an oxide of carbon (an oxocarbon), which can be viewed as the fourfold ketone of dioxane. It can also be viewed as the cyclic dimer of oxiranedione (C2O3), the hypothetical anhydride of oxalic acid.

Lithium chlorate is the inorganic chemical compound with the formula LiClO3. Like all chlorates, it is an oxidizer and may become unstable and possibly explosive if mixed with organic materials, reactive metal powders, or sulfur.
1,3-Dioxane or m-dioxane is a chemical compound with the molecular formula C4H8O2. It is a saturated six-membered heterocycle with two oxygen atoms in place of carbon atoms at the 1- and 3- positions. The corresponding five-membered rings are known as 1,3-dioxolanes.
Germanium dichloride dioxane is a chemical compound with the formula GeCl2(C4H8O2), where C4H8O2 is 1,4-dioxane. It is a white solid. The compound is notable as a source of Ge(II), which contrasts with the pervasiveness of Ge(IV) compounds. This dioxane complex represents a well-behaved form of germanium dichloride.