Related Research Articles

The regulation of therapeutic goods, defined as drugs and therapeutic devices, varies by jurisdiction. In some countries, such as the United States, they are regulated at the national level by a single agency. In other jurisdictions they are regulated at the state level, or at both state and national levels by various bodies, as in Australia.

The presence of the logo on commercial products indicates that the manufacturer or importer affirms the goods' conformity with European health, safety, and environmental protection standards. It is not a quality indicator or a certification mark. The CE marking is required for goods sold in the European Economic Area (EEA); goods sold elsewhere may also carry the mark.
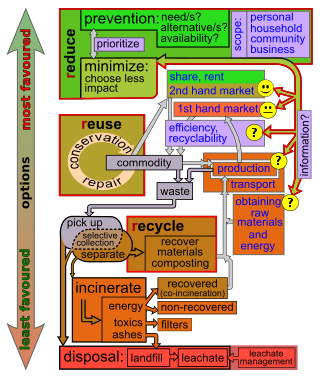
Waste (management) hierarchy is a tool used in the evaluation of processes that protect the environment alongside resource and energy consumption from most favourable to least favourable actions. The hierarchy establishes preferred program priorities based on sustainability. To be sustainable, waste management cannot be solved only with technical end-of-pipe solutions and an integrated approach is necessary.
The Undertakings for Collective Investment in Transferable Securities Directive (UCITS) 2009/65/EC is a consolidated EU directive that allows collective investment schemes to operate freely throughout the EU on the basis of a single authorisation from one member state. EU member states are entitled to have additional regulatory requirements for the benefit of investors.

EudraLex is the collection of rules and regulations governing medicinal products in the European Union.
Directive 75/318/EEC of 20 May 1975 on the approximation of the laws of Member States relating to analytical, pharmaco-toxicological and clinical standards and protocols in respect of the testing of proprietary medicinal products. This directive of the European Union sought to bring the benefits of innovative pharmaceuticals to patients across Europe by introducing the mutual recognition, by Member States, of their respective national marketing authorisations.
Council of the European Communities Directive 93/41/EEC of 14 June 1993 repealed Directive 87/22/EEC on the approximation of national measures relating to the marketing of high-technology medicinal products, as this directive had been superseded by Council Regulation (EEC) No 2309/93 of 22 July 1993 laying down Community procedures for the authorization and supervision of medicinal products for human and veterinary use and establishing a European Agency for the Evaluation of Medicinal Products (5) and by Council Directive 88/182/EEC of 22 March 1988 amending Directive 83/189/EEC laying down a procedure for the provision of information in the field of technical standards and regulations (6).
Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relates to medicinal products for human use in mainly countries that are part of the European Union. The Directive dealt with the disparities between certain national provisions, in particular between provisions relating to medicinal products, which directly affected the functioning of the internal market of the European Union.
The Clinical Trials Directive is a European Union directive that aimed at facilitating the internal market in medicinal products within the European Union, while at the same time maintaining an appropriate level of protection for public health. It seeks to simplify and harmonise the administrative provisions governing clinical trials in the European Community, by establishing a clear, transparent procedure.
The Good Clinical Practice Directive lays down principles and detailed guidelines for good clinical practice as regards conducting clinical trials of medicinal products for human use, as well as the requirements for authorisation of the manufacturing or importation of such products.

The Directive 2006/66/EC of the European Parliament and of the Council of 6 September 2006 on batteries and accumulators and waste batteries and accumulators and repealing Directive 91/157/EEC, commonly known as the Battery Directive, regulates the manufacture and disposal of batteries in the European Union with the aim of "improving the environmental performance of batteries and accumulators".

The Control of Substances Hazardous to Health Regulations 2002 is a United Kingdom Statutory Instrument which states general requirements imposed on employers to protect employees and other persons from the hazards of substances used at work by risk assessment, control of exposure, health surveillance and incident planning. There are also duties on employees to take care of their own exposure to hazardous substances and prohibitions on the import of certain substances into the European Economic Area. The regulations reenacted, with amendments, the Control of Substances Hazardous to Work Regulations 1999 and implement several European Union directives.

As of 2009, the European Union had issued two units of measurement directives. In 1971, it issued Directive 71/354/EEC, which required EU member states to standardise on the International System of Units (SI) rather than use a variety of CGS and MKS units then in use. The second, which replaced the first, was Directive 80/181/EEC, enacted in 1979 and later amended several times, which issued a number of derogations to the United Kingdom and Ireland based on the former directive.

The Product Liability Directive85/374/EEC is a directive of the Council of the European Communities which created a regime of strict liability for defective products applicable in all member states of the European Union, the other EEA members and the United Kingdom.
European labour law regulates basic transnational standards of employment and partnership at work in the European Union and countries adhering to the European Convention on Human Rights. In setting regulatory floors to competition for job-creating investment within the Union, and in promoting a degree of employee consultation in the workplace, European labour law is viewed as a pillar of the "European social model". Despite wide variation in employment protection and related welfare provision between member states, a contrast is typically drawn with conditions in the United States.

The Urban Waste Water Treatment Directive 1991 European Union directive concerning urban waste water "collection, treatment and discharge of urban waste water and the treatment and discharge of waste water from certain industrial sectors". It aims "to protect the environment from adverse effects of waste water discharges from cities and "certain industrial sectors". Council Directive 91/271/EEC on Urban Wastewater Treatment was adopted on 21 May 1991, amended by the Commission Directive 98/15/EC.

The Waste Framework Directive (WFD) is a European Union Directive concerned with "measures to protect the environment and human health by preventing or reducing the adverse impacts of the generation and management of waste and by reducing overall impacts of resource use and improving the efficiency of such use". The first Waste Framework Directive dates back to 1975. It had previously been substantially amended in 1991 and 2006. The present directive was adopted on 19 November 2008.

European company law is the part of European Union law which concerns the formation, operation and insolvency of companies in the European Union. The EU creates minimum standards for companies throughout the EU, and has its own corporate forms. All member states continue to operate separate companies acts, which are amended from time to time to comply with EU Directives and Regulations. There is, however, also the option of businesses to incorporate as a Societas Europaea (SE), which allows a company to operate across all member states.

Directive 2010/63/EU is the European Union (EU) legislation "on the protection of animals used for scientific purposes" and is one of the most stringent ethical and welfare standards worldwide.