Human cloning is the creation of a genetically identical copy of a human. The term is generally used to refer to artificial human cloning, which is the reproduction of human cells and tissue. It does not refer to the natural conception and delivery of identical twins. The possibility of human cloning has raised controversies. These ethical concerns have prompted several nations to pass laws regarding human cloning and its legality.
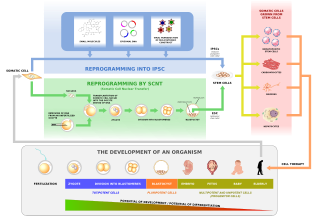
Stem cells are cells that can differentiate into other types of cells, and can also divide in self-renewal to produce more of the same type of stem cells.

Embryonic stem cells are pluripotent stem cells derived from the inner cell mass of a blastocyst, an early-stage pre-implantation embryo. Human embryos reach the blastocyst stage 4–5 days post fertilization, at which time they consist of 50–150 cells. Isolating the embryoblast, or inner cell mass (ICM) results in destruction of the blastocyst, a process which raises ethical issues, including whether or not embryos at the pre-implantation stage should have the same moral considerations as embryos in the post-implantation stage of development.
The regulation of science refers to use of law, or other ruling, by academic or governmental bodies to allow or restrict science from performing certain practices, or researching certain scientific areas.
The Dickey-Wicker Amendment is the name of an appropriation bill rider attached to a bill passed by United States Congress in 1995, and signed by former President Bill Clinton, which prohibits the Department of Health and Human Services (HHS) from using appropriated funds for the creation of human embryos for research purposes or for research in which human embryos are destroyed. HHS funding includes the funding for the National Institutes of Health (NIH). Technically the Dickey Amendment is a "rider" to other legislation, which amends the original legislation. The rider receives its name from the name of the Congressman that originally introduced the amendment, Representative Jay Dickey. The Dickey amendment language has been added to each of the Labor, HHS, and Education appropriations acts for FY1997 through FY2009. The original rider can be found in Section 128 of P.L. 104-99 . The wording of the rider is generally the same year after year. For FY2009, the wording in Division F, Section 509 of the Omnibus Appropriations Act, 2009, prohibits HHS, including NIH, from using FY2009 appropriated funds as follows:

James Alexander Thomson is an American developmental biologist best known for deriving the first human embryonic stem cell line in 1998 and for deriving human induced pluripotent stem cells (iPS) in 2007.
An institutional review board (IRB), also known as an independent ethics committee (IEC), ethical review board (ERB), or research ethics board (REB), is a type of committee that applies research ethics by reviewing the methods proposed for research to ensure that they are ethical. Such boards are formally designated to approve, monitor, and review biomedical and behavioral research involving humans. They often conduct some form of risk-benefit analysis in an attempt to determine whether or not research should be conducted. The purpose of the IRB is to assure that appropriate steps are taken to protect the rights and welfare of humans participating as subjects in a research study. Along with developed countries, many developing countries have established national, regional or local Institutional Review Boards in order to safeguard ethical conduct of research concerning both national and international norms, regulations or codes.
A stem cell line is a group of stem cells that is cultured in vitro and can be propagated indefinitely. Stem cell lines are derived from either animal or human tissues and come from one of three sources: embryonic stem cells, adult stem cells, or induced stem cells. They are commonly used in research and regenerative medicine. The simple formula to find out the length of this cell is x2 +yz5

Proposition 71 of 2004 is a law enacted by California voters to support stem cell research in the state. It was proposed by means of the initiative process and approved in the 2004 state elections on November 2. The Act amended both the Constitution of California and the Health and Safety Code.
The stem cell controversy is the consideration of the ethics of research involving the development, and use of human embryos. Most commonly, this controversy focuses on embryonic stem cells. Not all stem cell research involves human embryos. For example, adult stem cells, amniotic stem cells, and induced pluripotent stem cells do not involve creating, using, or destroying human embryos, and thus are minimally, if at all, controversial. Many less controversial sources of acquiring stem cells include using cells from the umbilical cord, breast milk, and bone marrow, which are not pluripotent.
Stem cell research policy varies significantly throughout the world. There are overlapping jurisdictions of international organizations, nations, and states or provinces. Some government policies determine what is allowed versus prohibited, whereas others outline what research can be publicly financed. Of course, all practices not prohibited are implicitly permitted. Some organizations have issued recommended guidelines for how stem cell research is to be conducted.

Ann A. Kiessling is an American reproductive biologist and one of the leaders in human parthenogenic stem cell research at The Bedford Research Foundation. She was an Associate Professor in teaching hospitals of Harvard Medical School from 1985 until 2012.
Kevin Eggan is a Professor of Stem Cell and Regenerative Biology at Harvard University, known for his work in stem cell research, and as a spokesperson for stem cell research in the United States. He was a 2006 recipient of a MacArthur Fellowship. In 2005, he was named to the MIT Technology Review TR35 as one of the top 35 innovators in the world under the age of 35.
An ethics committee is a body responsible for ensuring that medical experimentation and human subject research are carried out in an ethical manner in accordance with national and international law.
Stem cell laws are the law rules, and policy governance concerning the sources, research, and uses in treatment of stem cells in humans. These laws have been the source of much controversy and vary significantly by country. In the European Union, stem cell research using the human embryo is permitted in Sweden, Spain, Finland, Belgium, Greece, Britain, Denmark and the Netherlands; however, it is illegal in Germany, Austria, Ireland, Italy, and Portugal. The issue has similarly divided the United States, with several states enforcing a complete ban and others giving support. Elsewhere, Japan, India, Iran, Israel, South Korea, China, and Australia are supportive. However, New Zealand, most of Africa, and most of South America are restrictive.
Stem cell laws and policy in the United States have had a complicated legal and political history.
The laws and policies regarding stem cell research in the People's Republic of China are relatively relaxed in comparison to that of other nations. The reason for this is due to different traditional and cultural views in relation to that of the West.
George Q. Daley, is the Dean of the Faculty of Medicine, Caroline Shields Walker Professor of Medicine, and Professor of Biological Chemistry and Molecular Pharmacology at Harvard Medical School. He was formerly the Robert A. Stranahan Professor of Pediatrics at Harvard Medical School, Director of the Stem Cell Transplantation Program at Boston Children’s Hospital, and an investigator of the Howard Hughes Medical Institute, Associate Director of Children’s Stem Cell Program, a member of the Executive Committee of the Harvard Stem Cell Institute. He is a past president of the International Society for Stem Cell Research (2007–2008).
The International Society for Stem Cell Research (ISSCR) is an independent 501(c)(3) nonprofit organization based in Skokie, Illinois, United States. The organization's mission is to promote excellence in stem cell science and applications to human health.

Maneesha S. Inamdar is a developmental biologist specializing in stem cell research. She is a Professor and Chair at the Molecular Biology and Genetics Unit of the Jawaharlal Nehru Centre for Advanced Scientific Research (JNCASR). She was as an adjunct faculty at the Institute for Stem Cell Biology and Regenerative Medicine (inStem) and is an elected fellow of the Indian Academy of Sciences and the Indian National Science Academy.