
Estradiol (E2), also spelled oestradiol, is an estrogen steroid hormone and the major female sex hormone. It is involved in the regulation of the estrous and menstrual female reproductive cycles. Estradiol is responsible for the development of female secondary sexual characteristics such as the breasts, widening of the hips and a female-associated pattern of fat distribution. It is also important in the development and maintenance of female reproductive tissues such as the mammary glands, uterus and vagina during puberty, adulthood and pregnancy. It also has important effects in many other tissues including bone, fat, skin, liver, and the brain.

Estriol (E3), also spelled oestriol, is a steroid, a weak estrogen, and a minor female sex hormone. It is one of three major endogenous estrogens, the others being estradiol and estrone. Levels of estriol in women who are not pregnant are almost undetectable. However, during pregnancy, estriol is synthesized in very high quantities by the placenta and is the most produced estrogen in the body by far, although circulating levels of estriol are similar to those of other estrogens due to a relatively high rate of metabolism and excretion. Relative to estradiol, both estriol and estrone have far weaker activity as estrogens.
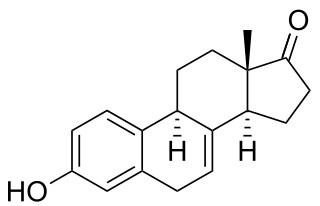
Equilin is a naturally occurring estrogen sex hormone found in horses as well as a medication. It is one of the estrogens present in the estrogen mixtures known as conjugated estrogens and esterified estrogens. CEEs is the most commonly used form of estrogen in hormone replacement therapy (HRT) for menopausal symptoms in the United States. Estrone sulfate is the major estrogen in CEEs while equilin sulfate is the second major estrogen in the formulation, present as about 25% of the total.
Estradiol valerate (EV), sold for use by mouth under the brand name Progynova and Primiwal E4 and for use by injection under the brand names Delestrogen and Progynon Depot among others, is an estrogen medication. It is used in hormone therapy for menopausal symptoms and low estrogen levels, hormone therapy for transgender people, and in hormonal birth control. It is also used in the treatment of prostate cancer. The medication is taken by mouth or by injection into muscle or fat once every 1 to 4 weeks.

Estradiol benzoate (EB), sold under the brand name Progynon-B among others, is an estrogen medication which is used in hormone therapy for menopausal symptoms and low estrogen levels in women, in hormone therapy for transgender women, and in the treatment of gynecological disorders. It is also used in the treatment of prostate cancer in men. Estradiol benzoate is used in veterinary medicine as well. When used clinically, the medication is given by injection into muscle usually two to three times per week.

Nomegestrol acetate (NOMAC), sold under the brand names Lutenyl and Zoely among others, is a progestin medication which is used in birth control pills, menopausal hormone therapy, and for the treatment of gynecological disorders. It is available both alone and in combination with an estrogen. NOMAC is taken by mouth. A birth control implant for placement under the skin was also developed but ultimately was not marketed.

Esterified estrogens (EEs), sold under the brand names Estratab and Menest among others, is an estrogen medication which is used hormone therapy for menopausal symptoms and low sex hormone levels in women, to treat breast cancer in both women and men, and to treat prostate cancer in men. It is formulated alone or in combination with methyltestosterone. It is taken by mouth.

Hydroxyprogesterone heptanoate (OHPH), also known as hydroxyprogesterone enanthate (OHPE) and sold under the brand names H.O.P., Lutogil A.P., and Lutogyl A.P. among others, is a progestin medication used for progestogenic indications. It has been formulated both alone and in together with estrogens, androgens/anabolic steroids, and other progestogens in several combination preparations. OHPH is given by injection into muscle at regular intervals.

Conjugated estrogens (CEs), or conjugated equine estrogens (CEEs), sold under the brand name Premarin among others, is an estrogen medication which is used in menopausal hormone therapy and for various other indications. It is a mixture of the sodium salts of estrogen conjugates found in horses, such as estrone sulfate and equilin sulfate. CEEs are available in the form of both natural preparations manufactured from the urine of pregnant mares and fully synthetic replications of the natural preparations. They are formulated both alone and in combination with progestins such as medroxyprogesterone acetate. CEEs are usually taken by mouth, but can also be given by application to the skin or vagina as a cream or by injection into a blood vessel or muscle.

Estradiol pivalate, also known as estradiol trimethyl acetate (E2-TMA) and sold under the brand name Estrotate, is an estrogen medication and an estrogen ester; specifically, a pivalic acid ester of estradiol. Literature sources are conflicting as to whether the ester is located at the C3 position or at the C17β position. It was marketed as an oil solution for intramuscular injection in the 1940s and 1950s. A combination of estradiol pivalate (1 mg/mL) and progesterone (10 mg/mL) in oil solution for intramuscular injection was available in 1949. An aqueous suspension of estradiol pivalate was also developed by 1950 although whether it was ever marketed is unclear.

Estradiol/progesterone (E2/P4), sold under the brand names Bijuva and Juvenum, is a combined estrogen and progestogen medication which is used in the treatment of menopausal symptoms in postmenopausal women. It contains estradiol, an estrogen, and progesterone, a progestogen, and is available in both oral and intramuscular formulations. E2/P4 differs from other estrogen–progestogen formulations in that the sex-hormonal agents used are bioidentical.

Estradiol (E2) is a medication and naturally occurring steroid hormone. It is an estrogen and is used mainly in menopausal hormone therapy and to treat low sex hormone levels in women. It is also used in hormonal birth control for women, in hormone therapy for transgender women, and in the treatment of hormone-sensitive cancers like prostate cancer in men and breast cancer in women, among other uses. Estradiol can be taken by mouth, held and dissolved under the tongue, as a gel or patch that is applied to the skin, in through the vagina, by injection into muscle or fat, or through the use of an implant that is placed into fat, among other routes.

Estrone (E1), sold under the brand names Estragyn, Kestrin, and Theelin among many others, is an estrogen medication and naturally occurring steroid hormone which has been used in menopausal hormone therapy and for other indications. It has been provided as an aqueous suspension or oil solution given by injection into muscle and as a vaginal cream applied inside of the vagina. It can also be taken by mouth as estradiol/estrone/estriol and in the form of prodrugs like estropipate and conjugated estrogens.

The pharmacology of estradiol, an estrogen medication and naturally occurring steroid hormone, concerns its pharmacodynamics, pharmacokinetics, and various routes of administration.

Estrone/progesterone (E1/P4), sold under the brand name Synergon, is a combination medication formulation of estrone, an estrogen, and progesterone, a progestogen, which is used as an injectable preparation to induce withdrawal bleeding in women with non-pregnancy-related amenorrhea . It has also sometimes been used off-label as an abortifacient. The medication comes in a three-ampoule pack, contains 1 mg estrone and 10 mg progesterone per ampoule, and is administered by intramuscular injection. The usual dose of the medication is three injections each two days apart, with the treatment duration not exceeding one week. E1/P4 is or has been available in France, Monaco, and Turkey, as well as in some French-speaking African countries such as Benin and Cameroon. The medication has been marketed since at least 1952.

Estradiol pivalate/progesterone (ETMA/P4), sold under the brand name Estrotate with Progesterone, is a combination medication of estradiol pivalate, an estrogen, and progesterone (P4), a progestogen, which was used in menopausal hormone therapy and the treatment of gynecological disorders but is no longer available. It contained 1 mg/mL ETMA and 10 mg/mL P4 in oil solution provided in vials and was administered by intramuscular injection at regular intervals.
Estrone/progesterone/testosterone (E1/P4/T), sold under the brand name Tristeron or Tristerone, is an injectable combination medication of estrone (E1), an estrogen, progesterone (P4), a progestogen, and testosterone (T), an androgen/anabolic steroid, which was used in the treatment of functional uterine bleeding in women. It contained 6 mg estrone, 50 mg progesterone, and 25 mg testosterone in microcrystalline aqueous suspension and was administered by intramuscular injection. The medication was manufactured by Wyeth and was marketed by 1951. It is no longer available.
Estradiol benzoate/progesterone/testosterone propionate (EB/P4/TP), sold under the brand names Lukestra, Steratrin, Trihormonal, and Trinestryl, is an injectable combination medication of estradiol benzoate (EB), an estrogen, progesterone (P4), a progestogen, and testosterone propionate (TP), an androgen/anabolic steroid. It contained 1 to 3 mg EB, 20 to 25 mg P4, and 25 mg TP, was provided in the form of ampoules, and was administered by intramuscular injection. The medication was introduced by 1949 and was marketed in the United States, the United Kingdom, and Germany among other places. It is no longer available.











