
Estradiol valerate (EV), sold for use by mouth under the brand name Progynova and for use by injection under the brand names Delestrogen and Progynon Depot among others, is an estrogen medication. It is used in hormone therapy for menopausal symptoms and low estrogen levels, hormone therapy for transgender people, and in hormonal birth control. It is also used in the treatment of prostate cancer. The medication is taken by mouth or by injection into muscle or fat once every 1 to 4 weeks.
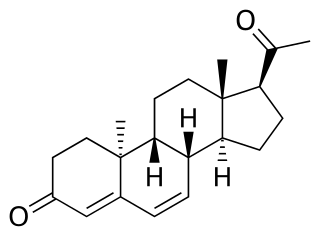
Dydrogesterone, sold under the brand name Duphaston among others, is a progestin medication which is used for a variety of indications, including threatened or recurrent miscarriage during pregnancy, dysfunctional bleeding, infertility due to luteal insufficiency, dysmenorrhea, endometriosis, secondary amenorrhea, irregular cycles, premenstrual syndrome, and as a component of menopausal hormone therapy. It is taken by mouth.

Hydroxyprogesterone caproate, sold under the brand names Proluton and Makena among others, is a medication used to reduce the risk of preterm birth in women pregnant with one baby who have a history of spontaneous preterm birth. In March 2023, the manufacturer, Covis Pharma, agreed to withdraw the drug from the US market. The approvals of Makena and its generics were withdrawn by the US Food and Drug Administration (FDA) in April 2023.

Estradiol benzoate (EB), sold under the brand name Progynon-B among others, is an estrogen medication which is used in hormone therapy for menopausal symptoms and low estrogen levels in women, in hormone therapy for transgender women, and in the treatment of gynecological disorders. It is also used in the treatment of prostate cancer in men. Estradiol benzoate is used in veterinary medicine as well. When used clinically, the medication is given by injection into muscle usually two to three times per week.

Medroxyprogesterone acetate (MPA), also known as depot medroxyprogesterone acetate (DMPA) in injectable form and sold under the brand name Depo-Provera among others, is a hormonal medication of the progestin type. It is used as a method of birth control and as a part of menopausal hormone therapy. It is also used to treat endometriosis, abnormal uterine bleeding, paraphilia, and certain types of cancer. The medication is available both alone and in combination with an estrogen. It is taken by mouth, used under the tongue, or by injection into a muscle or fat.

Estradiol cypionate (EC), sold under the brand name Depo-Estradiol among others, is an estrogen medication which is used in hormone therapy for menopausal symptoms and low estrogen levels in women, in hormone therapy for trans women, and in hormonal birth control for women. It is given by injection into muscle once every 1 to 4 weeks.
The progestogen challenge test, or progesterone withdrawal test, is a test used in the field of obstetrics and gynecology to evaluate a patient who is experiencing amenorrhea. Due to readily available assays to measure serum estradiol levels, this test is now rarely used.

Hydroxyprogesterone acetate (OHPA), sold under the brand name Prodox, is an orally active progestin related to hydroxyprogesterone caproate (OHPC) which has been used in clinical and veterinary medicine. It has reportedly also been used in birth control pills.

Estradiol/progesterone (E2/P4), sold under the brand name Bijuva among others, is a combined estrogen and progestogen medication which is used in the treatment of menopausal symptoms in postmenopausal women. It contains estradiol, an estrogen, and progesterone, a progestogen, and is available in both oral and intramuscular formulations. E2/P4 differs from other estrogen–progestogen formulations in that the sex-hormonal agents used are bioidentical.

Estrone (E1), sold under the brand names Estragyn, Kestrin, and Theelin among many others, is an estrogen medication and naturally occurring steroid hormone which has been used in menopausal hormone therapy and for other indications. It has been provided as an aqueous suspension or oil solution given by injection into muscle and as a vaginal cream applied inside of the vagina. It can also be taken by mouth as estradiol/estrone/estriol and in the form of prodrugs like estropipate and conjugated estrogens.

Estradiol benzoate/progesterone (EB/P4), sold under the brand names Duogynon and Sistocyclin among others, is a combination medication of estradiol benzoate (EB), an estrogen, and progesterone (P4), a progestogen. It has been formulated both as short-acting oil solutions and long-acting microcrystalline aqueous suspensions and is given by injection into muscle either once or continuously at regular intervals.

Estradiol valerate/hydroxyprogesterone caproate (EV/OHPC), sold under the brand names Gravibinon and Injectable No. 1 among others, is a combined estrogen and progestogen medication which is used in the treatment of threatened miscarriage and other indications and as a form of combined injectable birth control to prevent pregnancy. It contains estradiol valerate (EV), an estrogen, and hydroxyprogesterone caproate (OHPC), a progestin. The medication is given by injection into muscle once a day to once a month depending on the indication.

The pharmacokinetics of progesterone, concerns the pharmacodynamics, pharmacokinetics, and various routes of administration of progesterone.

Estradiol hemisuccinate/progesterone (EHS/P4), sold under the brand name Hosterona, is an injectable combination medication of estradiol hemisuccinate (EHS), an estrogen, and progesterone (P4), a progestogen, which is used to induce withdrawal bleeding in women with non-pregnancy-related amenorrhea. It is provided in the form of ampoules containing 20 mg/2 mL EHS and 100 mg/2 mL P4 and is administered by intramuscular injection. The medication is available in Argentina and Paraguay.

Estradiol benzoate/estradiol phenylpropionate (EB/EPP), sold under the brand name Dimenformon Prolongatum, is an injectable combination formulation of estradiol benzoate (EB), a shorter-acting estrogen, and estradiol phenylpropionate (EPP), a longer-acting estrogen, which has been used in menopausal hormone therapy for women in Europe but appears to no longer be available. It has also been used to suppress lactation in women and has been used in feminizing hormone therapy for transgender women. It has been provided in the form of 1 mL ampoules containing 2.5 mg EB and 10 mg EPP in oil solution and is administered by intramuscular injection at regular intervals.

Estradiol cypionate/hydroxyprogesterone caproate (EC/OHPC), sold under the brand name Sinbios, is a combination medication of estradiol cypionate (EC), an estrogen, and hydroxyprogesterone caproate (OHPC), a progestin, which was reportedly used as a combined injectable contraceptive in women in the early 1970s. It contained 5 mg EC and 250 mg OHPC in oil solution, was provided in the form of 1 mL ampoules, and was administered by intramuscular injection at regular intervals. The medication was manufactured by the pharmaceutical company Mavi in Mexico.
Estradiol benzoate/estradiol valerate/hydroxyprogesterone caproate (EB/EV/OHPC), sold under the brand name Sin-Ol, is a combination medication of estradiol benzoate (EB), an estrogen, estradiol valerate (EV), an estrogen, and hydroxyprogesterone caproate (OHPC), a progestin, which was reportedly used as a combined injectable contraceptive in women in the early 1970s. It contained 1 mg EB, 10 mg EV, and 250 mg OHPC in oil solution, was provided in the form of 3 mL ampoules, and was administered by intramuscular injection at regular intervals. The medication was manufactured by the pharmaceutical company Reuffer in Mexico.
Estrone/progesterone/testosterone (E1/P4/T), sold under the brand name Tristeron or Tristerone, is an injectable combination medication of estrone (E1), an estrogen, progesterone (P4), a progestogen, and testosterone (T), an androgen/anabolic steroid, which was used in the treatment of functional uterine bleeding in women. It contained 6 mg estrone, 50 mg progesterone, and 25 mg testosterone in microcrystalline aqueous suspension and was administered by intramuscular injection. The medication was manufactured by Wyeth and was marketed by 1951. It is no longer available.
Estradiol benzoate/progesterone/methandriol dipropionate (EB/P4/MADP), sold under the brand name Progestandron (Organon), is an injectable combination medication of estradiol benzoate (EB), an estrogen, progesterone (P4), a progestogen, and methandriol dipropionate (MADP), an androgen/anabolic steroid. It contained 3 mg EB, 20 mg P4, and 50 mg MADP, was provided in the form of ampoules, and was administered by intramuscular injection. The medication was marketed by 1957. It is no longer available.
Estradiol benzoate/progesterone/testosterone propionate (EB/P4/TP), sold under the brand names Lukestra, Steratrin, Trihormonal, and Trinestryl, is an injectable combination medication of estradiol benzoate (EB), an estrogen, progesterone (P4), a progestogen, and testosterone propionate (TP), an androgen/anabolic steroid. It contained 1 to 3 mg EB, 20 to 25 mg P4, and 25 mg TP, was provided in the form of ampoules, and was administered by intramuscular injection. The medication was introduced by 1949 and was marketed in the United States, the United Kingdom, and Germany among other places. It is no longer available.












